COVID-19 Vaccines Adverse Reactions Reported to the Pharmacovigilance Unit of Beira Interior in Portugal
- PMID: 36233459
- PMCID: PMC9571682
- DOI: 10.3390/jcm11195591
COVID-19 Vaccines Adverse Reactions Reported to the Pharmacovigilance Unit of Beira Interior in Portugal
Abstract
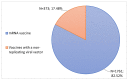
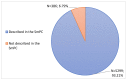
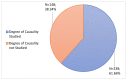
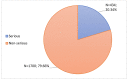
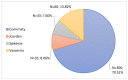
Coronavirus disease 2019 is an acute respiratory disease caused by the severe acute respiratory syndrome coronavirus 2. As the virus spreads rapidly, it has become a major public health emergency, which has led to rapid vaccines development. However, vaccines can present harmful and unintended responses, which must be notified to the National Pharmacovigilance System. The aim of this study is to characterize the adverse drug reactions (ADRs) of these vaccines notified in the region covered by the Regional Pharmacovigilance Unit (RPU) of Beira Interior, in Portugal, between 1 and 31 December 2020. During this period, 4 vaccines were administered: Comirnaty®, Spikevax®, Vaxzevria® and Jcovden®. The RPU of Beira Interior received 2134 notifications corresponding to 5685 ADRs, of which 20.34% (n = 434) of the notifications were considered serious reactions. Of these, 9.52% (n = 42) resulted in hospitalization and 0.45% (n = 2) resulted in death. Among the ADRs notified, reactions at or around the injection site, myalgia, headaches and pyrexia were the most commonly notified. Most ADRs were resolved within a few hours or days without sequelae. These ADRs are in accordance with clinical trials, the summary of product characteristics (SmPC) of each vaccine and ADR notifications from other countries. However, further studies are needed to confirm these results.
Keywords: COVID-19 vaccines; adverse drug reactions; immunization; mRNA vaccines; pharmacovigilance; safety; vaccines with a viral vector.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

