Variability of Isavuconazole Trough Concentrations during Longitudinal Therapeutic Drug Monitoring
- PMID: 36233624
- PMCID: PMC9573296
- DOI: 10.3390/jcm11195756
Variability of Isavuconazole Trough Concentrations during Longitudinal Therapeutic Drug Monitoring
Abstract
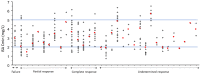
Isavuconazole (ISA), a triazole antifungal agent, is licensed for the treatment of invasive aspergillosis and mucormycosis. Therapeutic drug monitoring (TDM) is a cornerstone of treatment efficacy for triazole antifungals due to their pharmacokinetic variability, except for ISA, for which the utility of TDM is still uncertain. We performed a retrospective study that aimed to assess the inter- and intra-individual variability of ISA trough concentrations (Cmin) and to identify the determinants involved in such variability. ISA Cmin measured in adult patients at the Grenoble Alpes University Hospital between January 2018 and August 2020 were retrospectively analyzed. In total, 304 ISA Cmin for 33 patients were analyzed. The median ISA Cmin was 2.8 [25th−75th percentiles: 2.0−3.7] mg/L. The inter- and intra-individual variability was 41.5% and 30.7%, respectively. Multivariate analysis showed independent covariate effects of dose (β = 0.004 ± 3.56 × 10−4, p < 0.001), Aspartate aminotransférase (ASAT) (β = 0.002 ± 5.41 × 10−4, p = 0.002), and protein levels (β = 0.022 ± 0.004, p < 0.001) on ISA Cmin, whereas C reactive protein levels did not show any association. This study, conducted on a large number of ISA Cmin, shows that ISA exposure exhibits variability, explained in part by the ISA dose, and ASAT and protein levels.
Keywords: isavuconazole; pharmacokinetics; therapeutic drug monitoring; trough concentration.
Conflict of interest statement
Gautier-Veyret obtained personal fees from Gilead. Gautier-Veyret and Stanke-Labesque report grants from MSD, outside the scope of the work submitted, and grants from Pfizer for this study. Bolcato and Anne Thiebaut-Bertrand have nothing to disclose.
Figures

References
-
- Maertens J.A., Raad I.I., Marr K.A., Patterson T.F., Kontoyiannis D.P., Cornely O.A., Bow E.J., Rahav G., Neofytos D., Aoun M., et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): A phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387:760–769. doi: 10.1016/S0140-6736(15)01159-9. - DOI - PubMed
-
- Schmitt-Hoffmann A., Desai A., Kowalski D., Pearlman H., Yamazaki T., Townsend R. Isavuconazole absorption following oral administration in healthy subjects is comparable to intravenous dosing, and is not affected by food, or drugs that alter stomach pH. Int. J. Clin. Pharmacol. Ther. 2016;54:572–580. doi: 10.5414/CP202434. - DOI - PubMed
-
- Schmitt-Hoffmann A., Roos B., Maares J., Heep M., Spickerman J., Weidekamm E., Brown T., Roehrle M. Multiple-Dose Pharmacokinetics and Safety of the New Antifungal Triazole BAL4815 after Intravenous Infusion and Oral Administration of Its Prodrug, BAL8557, in Healthy Volunteers. Antimicrob. Agents Chemother. 2006;50:286–293. doi: 10.1128/AAC.50.1.286-293.2006. - DOI - PMC - PubMed
-
- Jović Z., Janković S.M., Ružić Zečević D., Milovanović D., Stefanović S., Folić M., Milovanović J., Kostić M. Clinical Pharmacokinetics of Second-Generation Triazoles for the Treatment of Invasive Aspergillosis and Candidiasis. Eur. J. Drug Metab. Pharmacokinet. 2019;44:139–157. doi: 10.1007/s13318-018-0513-7. - DOI - PubMed
-
- Ullmann A.J., Aguado J.M., Arikan-Akdagli S., Denning D.W., Groll A.H., Lagrou K., Lass-Flörl C., Lewis R.E., Munoz P., Verweij P.E., et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018;24((Suppl. S1)):e1–e38. doi: 10.1016/j.cmi.2018.01.002. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

