Gold-Nanoparticle-Coated Magnetic Beads for ALP-Enzyme-Based Electrochemical Immunosensing in Human Plasma
- PMID: 36234217
- PMCID: PMC9573121
- DOI: 10.3390/ma15196875
Gold-Nanoparticle-Coated Magnetic Beads for ALP-Enzyme-Based Electrochemical Immunosensing in Human Plasma
Abstract
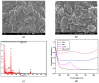
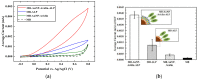
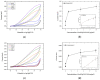
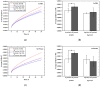
A simple and sensitive AuNP-coated magnetic beads (AMB)-based electrochemical biosensor platform was fabricated for bioassay. In this study, AuNP-conjugated magnetic particles were successfully prepared using biotin-streptavidin conjugation. The morphology and structure of the nanocomplex were characterized by scanning electron microscopy (SEM) with energy-dispersive X-ray analysis (EDX) and UV-visible spectroscopy. Moreover, cyclic voltammetry (CV) was used to investigate the effect of AuNP-MB on alkaline phosphatase (ALP) for electrochemical signal enhancement. An ALP-based electrochemical (EC) immunoassay was performed on the developed AuNP-MB complex with indium tin oxide (ITO) electrodes. Subsequently, the concentration of capture antibodies was well-optimized on the AMB complex via biotin-avidin conjugation. Lastly, the developed AuNP-MB immunoassay platform was verified with extracellular vesicle (EV) detection via immune response by showing the existence of EGFR proteins on glioblastoma multiforme (GBM)-derived EVs (108 particle/mL) spiked in human plasma. Therefore, the signal-enhanced ALP-based EC biosensor on AuNP-MB was favorably utilized as an immunoassay platform, revealing the potential application of biosensors in immunoassays in biological environments.
Keywords: electrochemical immunoassay; gold nanoparticle; human plasma; indium tin oxide; magnetic bead.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

