Pseudocapacitive Effects of Multi-Walled Carbon Nanotubes-Functionalised Spinel Copper Manganese Oxide
- PMID: 36234643
- PMCID: PMC9565235
- DOI: 10.3390/nano12193514
Pseudocapacitive Effects of Multi-Walled Carbon Nanotubes-Functionalised Spinel Copper Manganese Oxide
Abstract
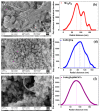
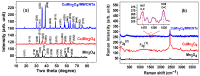
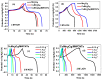
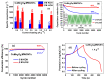
Spinel copper manganese oxide nanoparticles combined with acid-treated multi-walled carbon nanotubes (CuMn2O4/MWCNTs) were used in the development of electrodes for pseudocapacitor applications. The CuMn2O4/MWCNTs preparation involved initial synthesis of Mn3O4 and CuMn2O4 precursors followed by an energy efficient reflux growth method for the CuMn2O4/MWCNTs. The CuMn2O4/MWCNTs in a three-electrode cell assembly and in 3 M LiOH aqueous electrolyte exhibited a specific capacitance of 1652.91 F g-1 at 0.5 A g-1 current load. Similar investigation in 3 M KOH aqueous electrolyte delivered a specific capacitance of 653.41 F g-1 at 0.5 A g-1 current load. Stability studies showed that after 6000 cycles, the CuMn2O4/MWCNTs electrode exhibited a higher capacitance retention (88%) in LiOH than in KOH (64%). The higher capacitance retention and cycling stability with a Coulombic efficiency of 99.6% observed in the LiOH is an indication of a better charge storage behaviour in this electrolyte than in the KOH electrolyte with a Coulombic efficiency of 97.3%. This superior performance in the LiOH electrolyte than in the KOH electrolyte is attributed to an intercalation/de-intercalation mechanism which occurs more easily in the LiOH electrolyte than in the KOH electrolyte.
Keywords: galvanostatic charge/discharge; nanocomposite electrode; pseudocapacitor; specific capacitance; spinel metal oxide.
Conflict of interest statement
The authors do not have any conflict of interest to declare.
Figures









References
-
- Chen Y., Zhang X., Xu C., Xu H. The fabrication of asymmetry supercapacitor based on MWCNTs/MnO2/PPy composites. Electrochim. Acta. 2019;309:424–431. doi: 10.1016/j.electacta.2019.04.072. - DOI
-
- Pratibha G., Srinivas I., Rao K.V., Shanker A.K., Raju B.M.K., Choudhary D.K., Rao K.S., Srinivasarao C., Maheswari M. Net global warming potential and greenhouse gas intensity of conventional and conservation agriculture system in rainfed semi-arid tropics of India. Atmos. Environ. 2016;145:239–250. doi: 10.1016/j.atmosenv.2016.09.039. - DOI
-
- Zhang H., Li H., Sun Z., Jia D. One-step hydrothermal synthesis of NiCo2S4 nanoplates/nitrogen-doped mesoporous carbon composites as advanced electrodes for asymmetric supercapacitors. J. Power Sources. 2019;439:227082. doi: 10.1016/j.jpowsour.2019.227082. - DOI
-
- Akhtar M.S., Gul I.H., Baig M.M., Akram M.A. Binder-free pseudocapacitive nickel cobalt sulfide/MWCNTs hybrid electrode directly grown on nickel foam for high rate supercapacitors. Mater. Sci. Eng. B. 2021;264:114898. doi: 10.1016/j.mseb.2020.114898. - DOI
-
- Rajkumar S., Elanthamilan E., Balaji T.E., Sathiyan A., Jafneel N.E., Merlin J.P. Recovery of copper oxide nanoparticles from waste SIM cards for supercapacitor electrode material. J. Alloy. Compd. 2020;849:156582. doi: 10.1016/j.jallcom.2020.156582. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

