Advocacy for the Medicinal Plant Artabotrys hexapetalus (Yingzhao) and Antimalarial Yingzhaosu Endoperoxides
- PMID: 36234725
- PMCID: PMC9573098
- DOI: 10.3390/molecules27196192
Advocacy for the Medicinal Plant Artabotrys hexapetalus (Yingzhao) and Antimalarial Yingzhaosu Endoperoxides
Abstract
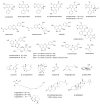
The medicinal plant Artabotrys hexapetalus (synonyms: A.uncinatus and A. odoratissimus) is known as yingzhao in Chinese. Extracts of the plant have long been used in Asian folk medicine to treat various symptoms and diseases, including fevers, microbial infections, ulcers, hepatic disorders and other health problems. In particular, extracts from the roots and fruits of the plant are used for treating malaria. Numerous bioactive natural products have been isolated from the plant, mainly aporphine (artabonatines, artacinatine) and benzylisoquinoline (hexapetalines) alkaloids, terpenoids (artaboterpenoids), flavonoids (artabotrysides), butanolides (uncinine, artapetalins) and a small series of endoperoxides known as yingzhaosu A-to-D. These natural products confer antioxidant, anti-inflammatory and antiproliferative properties to the plant extracts. The lead compound yingzhaosu A displays marked activities against the malaria parasites Plasmodium falciparum and P. berghei. Total syntheses have been developed to access yingzhaosu compounds and analogues, such as the potent compound C14-epi-yingzhaosu A and simpler molecules with a dioxane unit. The mechanism of action of yingzhaosu A points to an iron(II)-induced degradation leading to the formation of two alkylating species, an unsaturated ketone and a cyclohexyl radical, which can then react with vital parasitic proteins. A bioreductive activation of yingzhaosu A endoperoxide can also occur with the heme iron complex. The mechanism of action of yingzhaosu endoperoxides is discussed, to promote further chemical and pharmacological studies of these neglected, but highly interesting bioactive compounds. Yingzhaosu A/C represent useful templates for designing novel antimalarial drugs.
Keywords: Artabotrys hexapetalus; endoperoxide; malaria; natural products; yingzhaosu.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

