The First 5'-Phosphorylated 1,2,3-Triazolyl Nucleoside Analogues with Uracil and Quinazoline-2,4-Dione Moieties: A Synthesis and Antiviral Evaluation
- PMID: 36234748
- PMCID: PMC9573387
- DOI: 10.3390/molecules27196214
The First 5'-Phosphorylated 1,2,3-Triazolyl Nucleoside Analogues with Uracil and Quinazoline-2,4-Dione Moieties: A Synthesis and Antiviral Evaluation
Abstract
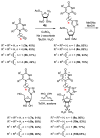
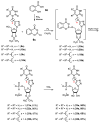
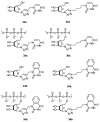
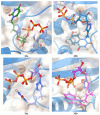
A series of 5'-phosphorylated (dialkyl phosphates, diaryl phosphates, phosphoramidates, H-phosphonates, phosphates) 1,2,3-triazolyl nucleoside analogues in which the 1,2,3-triazole-4-yl-β-D-ribofuranose fragment is attached via a methylene group or a butylene chain to the N-1 atom of the heterocycle moiety (uracil or quinazoline-2,4-dione) was synthesized. All compounds were evaluated for antiviral activity against influenza virus A/PR/8/34/(H1N1). Antiviral assays revealed three compounds, 13b, 14b, and 17a, which showed moderate activity against influenza virus A (H1N1) with IC50 values of 17.9 μM, 51 μM, and 25 μM, respectively. In the first two compounds, the quinazoline-2,4-dione moiety is attached via a methylene or a butylene linker, respectively, to the 1,2,3-triazole-4-yl-β-D-ribofuranosyl fragment possessing a 5'-diphenyl phosphate substituent. In compound 17a, the uracil moiety is attached via the methylene unit to the 1,2,3-triazole-4-yl-β-D-ribofuranosyl fragment possessing a 5'-(phenyl methoxy-L-alaninyl)phosphate substituent. The remaining compounds appeared to be inactive against influenza virus A/PR/8/34/(H1N1). The results of molecular docking simulations indirectly confirmed the literature data that the inhibition of viral replication is carried out not by nucleoside analogues themselves, but by their 5'-triphosphate derivatives.
Keywords: 1,2,3-triazole; antivirals; click chemistry; influenza virus; nucleoside analogues; nucleotides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



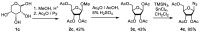


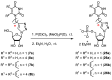
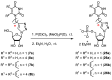
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

