Compositional Study of Phospholipids from the Dried Big Head and Opossum Shrimp, Mussel, and Sea Cucumber Using 31P NMR Spectroscopy: Content and Fatty Acid Composition of Plasmalogen
- PMID: 36234786
- PMCID: PMC9571261
- DOI: 10.3390/molecules27196250
Compositional Study of Phospholipids from the Dried Big Head and Opossum Shrimp, Mussel, and Sea Cucumber Using 31P NMR Spectroscopy: Content and Fatty Acid Composition of Plasmalogen
Abstract
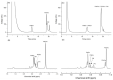
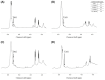
Herein, we present a qualitative and quantitative analysis of the compositions of plasmalogens and phospholipids (PLs) in dried big head shrimp (Solenocera melantho), opossum shrimp (Neomysis awatschensis), mussel (Mytilus galloprovincialis), and sea cucumber (Apostichopus japonicus). We also analyze the fatty acid composition of the extracted lipids, phosphatidyl choline (PtdCho), and plasmalogen choline (PlsCho) from each sample. In big head shrimp, opossum shrimp, and mussel, phosphatidyl choline (PtdCho) was the most abundant PL at 1677.9, 1603, and 1661.6 mg/100 g of dried sample, respectively, whereas the most abundant PL in sea cucumber was PlsCho (206.9 mg/100 g of dried sample). In all four samples, plasmalogen ethanolamine (PlsEtn) was higher than phosphatidyl ethanolamine (PtdEtn). The content (mg/100 g of dried sample) of PlsCho was highest in mussel (379.0), and it was higher in big head shrimp (262.3) and opossum shrimp (245.6) than sea cucumber (206.9). The contents (mg/100 g of dried sample) of PlsEtn were in the order of mussel (675.4) > big head shrimp (629.5) > opossum shrimp (217.9) > sea cucumber (51.5). For analyzing the fatty acids at the sn-2 position of PlsCho, the consecutive treatment with phospholipase A1, solid phase extraction, thin-layer chromatography (TLC), and GC-FID were applied. The most abundant fatty acid was eicosapentaenoic acid (EPA, C20:5, n-3) in big head shrimp and sea cucumber, palmitoleic acid (C16:1, n-7) in opossum shrimp, and docosadienoic acid (C22:2, n-6) in mussel.
Keywords: 31P-NMR; dried marine animal; phospholipids; plasmalogen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Yamashita S., Kiko T., Fujiwara H., Hashimoto M., Nakagawa K., Kinoshita M., Furukawa K., Arai H., Miyazawa T. Alterations in the levels of amyloid-β, phospholipid hydroperoxide, and plasmalogen in the blood of patients with Alzheimer’s disease: Possible interactions between amyloid-β and these lipids. J. Alzheimer’s Dis. 2016;50:527–537. doi: 10.3233/JAD-150640. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

