Effects of Environmental and Electric Perturbations on the pKa of Thioredoxin Cysteine 35: A Computational Study
- PMID: 36234991
- PMCID: PMC9570579
- DOI: 10.3390/molecules27196454
Effects of Environmental and Electric Perturbations on the pKa of Thioredoxin Cysteine 35: A Computational Study
Abstract
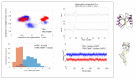
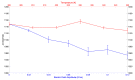
Here we present a theoretical-computational study dealing with the evaluation of the pKa of the Cysteine residues in Thioredoxin (TRX) and in its complex with the Thioredoxin-interacting protein (TXNIP). The free energy differences between the anionic and neutral form of the Cysteine 32 and 35 have been evaluated by means of the Perturbed Matrix Method with classical perturbations due to both the environment and an exogenous electric field as provided by Molecular Dynamics (MD) simulations. The evaluation of the free energies allowed us to show that the effect of the perturbing terms is to lower the pKa of Cysteine 32 and Cysteine 35 with respect to the free amino-acid. On the other hand, in the complex TRX-TXNIP, our data show an enhanced stabilization of the neutral reduced form of Cys 35. These results suggest that external electric stimuli higher than 0.02 V/nm can modulate the Cysteine pKa, which can be connected to the tight regulation of the TRX acting as an antioxidant agent.
Keywords: PMM; Thioredoxin; electric field; molecular communications; pKa.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

