Biomimetic Targeted Theranostic Nanoparticles for Breast Cancer Treatment
- PMID: 36235009
- PMCID: PMC9571674
- DOI: 10.3390/molecules27196473
Biomimetic Targeted Theranostic Nanoparticles for Breast Cancer Treatment
Abstract
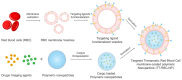
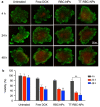
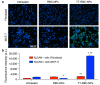
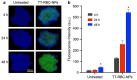
The development of biomimetic drug delivery systems for biomedical applications has attracted significant research attention. As the use of cell membrane as a surface coating has shown to be a promising platform for several disease treatments. Cell-membrane-coated nanoparticles exhibit enhanced immunocompatibility and prolonged circulation time. Herein, human red blood cell (RBC) membrane-cloaked nanoparticles with enhanced targeting functionality were designed as a targeted nanotheranostic against cancer. Naturally, derived human RBC membrane modified with targeting ligands coated onto polymeric nanoparticle cores containing both chemotherapy and imaging agent. Using epithelial cell adhesion molecule (EpCAM)-positive MCF-7 breast cancer cells as a disease model, the nature-inspired targeted theranostic human red blood cell membrane-coated polymeric nanoparticles (TT-RBC-NPs) platform was capable of not only specifically binding to targeted cancer cells, effectively delivering doxorubicin (DOX), but also visualizing the targeted cancer cells. The TT-RBC-NPs achieved an extended-release profile, with the majority of the drug release occurring within 5 days. The TT-RBC-NPs enabled enhanced cytotoxic efficacy against EpCAM positive MCF-7 breast cancer over the non-targeted NPs. Additionally, fluorescence images of the targeted cancer cells incubated with the TT-RBC-NPs visually indicated the increased cellular uptake of TT-RBC-NPs inside the breast cancer cells. Taken together, this TT-RBC-NP platform sets the foundation for the next-generation stealth theranostic platforms for systemic cargo delivery for treatment and diagnostic of cancer.
Keywords: biomimetic; cancer; nanomedicine; nanoparticles; theranostics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Curigliano G., Cardinale D., Suter T., Plataniotis G., De Azambuja E., Sandri M.T., Criscitiello C., Goldhirsch A., Cipolla C., Roila F. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 2012;23:vii155–vii166. doi: 10.1093/annonc/mds293. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

