Structural Diversity and Carbon Dioxide Sorption Selectivity of Zinc(II) Metal-Organic Frameworks Based on Bis(1,2,4-triazol-1-yl)methane and Terephthalic Acid
- PMID: 36235016
- PMCID: PMC9571910
- DOI: 10.3390/molecules27196481
Structural Diversity and Carbon Dioxide Sorption Selectivity of Zinc(II) Metal-Organic Frameworks Based on Bis(1,2,4-triazol-1-yl)methane and Terephthalic Acid
Abstract
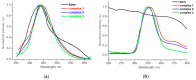
A three-component reaction between the 1,4-benzenedicarboxylic (terephthalic) acid (H2bdc), bis(1,2,4-triazol-1-yl)methane (btrm) and zinc nitrate was studied, and three new coordination polymers were isolated by a careful selection of the reaction conditions. Coordination polymers {[Zn3(DMF)(btrm)(bdc)3]·nDMF}∞ and {[Zn3(btrm)(bdc)3]·nDMF}∞ containing trinuclear {Zn3(bdc)3} secondary building units are joined by btrm auxiliary linkers into three-dimensional metal-organic frameworks. The coordination polymer {[Zn(bdc)(btrm)]∙nDMF}∞ consists of Zn2+ cations joined by bdc2- and btrm linkers into a two-fold interpenetrated network. Upon activation, MOF [Zn3(btrm)(bdc)3]∞ demonstrated CO2/N2 adsorption selectivity with an ideal adsorbed solution theory (IAST) factor of 21. All three MOF demonstrated photoluminescence with a maximum near 435-440 nm upon excitation at 330 nm.
Keywords: bis(1,2,4-triazol-1-yl)methane; coordination polymers; gas adsorption; gas separation; luminescence; metal–organic frameworks; terephthalic acid.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures







References
-
- Dubskikh V.A., Lysova A.A., Samsonenko D.G., Lavrov A.N., Kovalenko K.A., Dybtsev D.N., Fedin V.P. 3D Metal–Organic Frameworks Based on Co(II) and Bithiophendicarboxylate: Synthesis, Crystal Structures, Gas Adsorption, and Magnetic Properties. Molecules. 2021;26:1269. doi: 10.3390/molecules26051269. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

