Effect of Formic Acid on the Outdiffusion of Ti Interstitials at TiO2 Surfaces: A DFT+U Investigation
- PMID: 36235075
- PMCID: PMC9572331
- DOI: 10.3390/molecules27196538
Effect of Formic Acid on the Outdiffusion of Ti Interstitials at TiO2 Surfaces: A DFT+U Investigation
Abstract
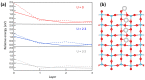
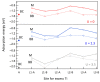
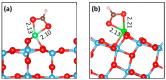
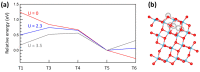
Ti interstitials play a key role in the surface chemistry of TiO2. However, because of their elusive behavior, proof of their participation in catalytic processes is difficult to obtain. Here, we used DFT+U calculations to investigate the interaction between formic acid (FA) and excess Ti atoms on the rutile-TiO2(110) and anatase-TiO2(101) surfaces. The excess Ti atoms favor FA dissociation, while decreasing the relative stability of the bidentate bridging coordination over the monodentate one. FA species interact significantly with the Ti interstitials, favoring their outdiffusion. Eventually, Ti atoms can emerge at the surface forming chelate species, which are more stable than monodentate FA species in the case of rutile, and are even energetically favored in the case of anatase. The presence of Ti adatoms that can directly participate to surface processes should then be considered when formic acid and possibly carboxylate-bearing species are adsorbed onto TiO2 particles.
Keywords: density functional theory; formic acid; interstitial defects; titanium dioxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Wandelt K. Properties and Influence of Surface Defects. Surf. Sci. 1991;251–252:387–395. doi: 10.1016/0039-6028(91)91021-O. - DOI
-
- Danish M.S.S., Bhattacharya A., Stepanova D., Mikhaylov A., Grilli M.L., Khosravy M., Senjyu T. A Systematic Review of Metal Oxide Applications for Energy and Environmental Sustainability. Metals. 2020;10:1604. doi: 10.3390/met10121604. - DOI
-
- Kazachenko A., Vasilieva N., Fetisova O., Sychev V., Elsuf’ev E., Malyar Y., Issaoui N., Miroshnikova A., Borovkova V., Kazachenko A., et al. New Reactions of Betulin with Sulfamic Acid and Ammonium Sulfamate in the Presence of Solid Catalysts. Biomass Convers. Biorefinery. 2022;1265:133394. doi: 10.1007/s13399-022-02587-x. - DOI
-
- Bennett R.A., McCavish N.D. Non-Stoichiometric Oxide Surfaces and Ultra-Thin Films: Characterisation of TiO2. Top. Catal. 2005;36:11–19. doi: 10.1007/s11244-005-7858-2. - DOI
LinkOut - more resources
Full Text Sources

