Effects of Donepezil Treatment on Brain Metabolites, Gut Microbiota, and Gut Metabolites in an Amyloid Beta-Induced Cognitive Impairment Mouse Pilot Model
- PMID: 36235127
- PMCID: PMC9572896
- DOI: 10.3390/molecules27196591
Effects of Donepezil Treatment on Brain Metabolites, Gut Microbiota, and Gut Metabolites in an Amyloid Beta-Induced Cognitive Impairment Mouse Pilot Model
Abstract
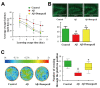
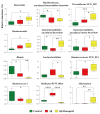
Accumulated clinical and biomedical evidence indicates that the gut microbiota and their metabolites affect brain function and behavior in various central nervous system disorders. This study was performed to investigate the changes in brain metabolites and composition of the fecal microbial community following injection of amyloid β (Aβ) and donepezil treatment of Aβ-injected mice using metataxonomics and metabolomics. Aβ treatment caused cognitive dysfunction, while donepezil resulted in the successful recovery of memory impairment. The Aβ + donepezil group showed a significantly higher relative abundance of Verrucomicrobia than the Aβ group. The relative abundance of 12 taxa, including Blautia and Akkermansia, differed significantly between the groups. The Aβ + donepezil group had higher levels of oxalate, glycerol, xylose, and palmitoleate in feces and oxalate, pyroglutamic acid, hypoxanthine, and inosine in brain tissues than the Aβ group. The levels of pyroglutamic acid, glutamic acid, and phenylalanine showed similar changes in vivo and in vitro using HT-22 cells. The major metabolic pathways in the brain tissues and gut microbiota affected by Aβ or donepezil treatment of Aβ-injected mice were related to amino acid pathways and sugar metabolism, respectively. These findings suggest that alterations in the gut microbiota might influence the induction and amelioration of Aβ-induced cognitive dysfunction via the gut-brain axis. This study could provide basic data on the effects of Aβ and donepezil on gut microbiota and metabolites in an Aβ-induced cognitive impairment mouse model.
Keywords: Alzheimer’s disease; amyloid beta; donepezil; gut microbiome; metabolite.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this study.
Figures




References
-
- Mahajan U.V., Varma V.R., Griswold M.E., Blackshear C.T., An Y., Oommen A.M., Varma S., Troncoso J.C., Pletnikova O., O’Brien R. Dysregulation of multiple metabolic networks related to brain transmethylation and polyamine pathways in Alzheimer disease: A targeted metabolomic and transcriptomic study. PLoS Med. 2020;17:e1003012. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

