Polymeric Emissive Materials Based on Dynamic Covalent Bonds
- PMID: 36235170
- PMCID: PMC9570607
- DOI: 10.3390/molecules27196635
Polymeric Emissive Materials Based on Dynamic Covalent Bonds
Abstract
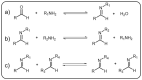
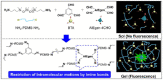
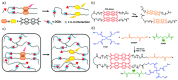
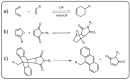
Dynamic covalent polymers, composed of dynamic covalent bonds (DCBs), have received increasing attention in the last decade due to their adaptive and reversible nature compared with common covalent linked polymers. Incorporating the DCBs into the polymeric material endows it with advanced performance including self-healing, shape memory property, and so forth. However, the emissive ability of such dynamic covalent polymeric materials has been rarely reviewed. Herein, this review has summarized DCBs-based emissive polymeric materials which are classified according to the different types of DCBs, including imine bond, acylhydrazone bond, boronic ester bond, dynamic C-C bond, as well as the reversible bonds based on Diels-Alder reaction and transesterification. The mechanism of chemical reactions and various stimuli-responsive behaviors of DCBs are introduced, followed by typical emissive polymers resulting from these DCBs. By taking advantage of the reversible nature of DCBs under chemical/physical stimuli, the constructed emissive polymeric materials show controllable and switchable emission. Finally, challenges and future trends in this field are briefly discussed in this review.
Keywords: dynamic covalent bond; dynamic covalent chemistry; polymeric emissive material; stimuli polymer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













References
-
- Zhang W., Li J.-X., Tang R.-C., Zhai A.-D. Hydrophilic and antibacterial surface functionalization of polyamide fabric by coating with polylysine biomolecule. Prog. Org. Coat. 2020;142:105571. doi: 10.1016/j.porgcoat.2020.105571. - DOI
-
- Kazmi S.M.S., Munir M.J., Wu Y.-F. Application of waste tire rubber and recycled aggregates in concrete products: A new compression casting approach. Resour. Conserv. Recycl. 2021;167:105353. doi: 10.1016/j.resconrec.2020.105353. - DOI
-
- Bei Y., Ma Y., Song F., Kou Z., Hu L., Bo C., Jia P., Zhou Y. Recent progress of biomass based self-healing polymers. J. Appl. Polym. Sci. 2022;139:51977. doi: 10.1002/app.51977. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

