NMR Investigation of the Interaction of Three Non-Steroidal Anti-Inflammatory Drugs with Human Serum Albumin
- PMID: 36235184
- PMCID: PMC9571845
- DOI: 10.3390/molecules27196647
NMR Investigation of the Interaction of Three Non-Steroidal Anti-Inflammatory Drugs with Human Serum Albumin
Abstract
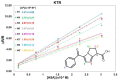
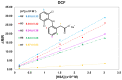
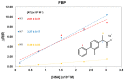
The understanding of the interaction between non-steroidal anti-inflammatory drugs and human serum albumin plays a fundamental role in the development of new drugs and new therapeutic strategies. Several studies have been performed, nevertheless, the interaction phenomena are still not fully understood. In this work, high-field solution Nuclear Magnetic Resonance (NMR) spectroscopy was applied to compare the strength of the interaction of diclofenac sodium salt, ketorolac tris salt and flurbiprofen sodium salt toward albumin. To this aim, mono- and bi-selective relaxation rate measurements were performed by applying selective π-pulses at the selected frequencies and by following magnetization recovery. On the basis of the dependence of relaxation parameters on albumin concentration, normalized affinity indexes were calculated for several protons of the drugs. Affinity indexes for diclofenac were about five-fold higher in comparison with ketorolac and flurbiprofen. Aromatic moieties of the three drugs and methine protons at the chiral centers of ketorolac and flurbiprofen were more involved in the interaction with albumin. In conclusion, NMR spectroscopy allows not only for the comparison of drug-to-protein affinities but also points out the nature of the drug sites that are more extensively involved in the interaction.
Keywords: NMR; NSAIDs; affinity index; binding; protein/molecule interaction; selective relaxation rates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

