Comparing Variants of the Cell-Penetrating Peptide sC18 to Design Peptide-Drug Conjugates
- PMID: 36235193
- PMCID: PMC9570898
- DOI: 10.3390/molecules27196656
Comparing Variants of the Cell-Penetrating Peptide sC18 to Design Peptide-Drug Conjugates
Abstract
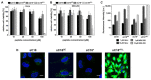
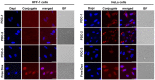
Herein, the design and synthesis of peptide-drug conjugates (PDCs) including different variants of the cell-penetrating peptide sC18 is presented. We first generated a series of novel sequence mutants of sC18 having either amino acid deletions and/or substitutions, and then tested their biological activity. The effects of histidine substituents were found to be not meaningful for sC18 uptake and cell selectivity. Moreover, building a nearly perfect amphipathic structure within a shortened sC18 derivative provided a peptide that was highly membrane-active, but also too cytotoxic. As a result, the most promising analog was sC18ΔE, which stands out due to its higher uptake efficacy compared to parent sC18. In the last set of experiments, we let the peptides react with the cytotoxic drug doxorubicin by Thiol-Michael addition to form novel PDCs. Our results indicate that sC18ΔE could be a more efficient drug carrier than parent sC18 for biomedical applications. However, cellular uptake using endocytosis and resulting entrapment of cargo inside vesicles is still a major critical step to overcome in CPP-containing peptide-drug development.
Keywords: cancer; cell-penetrating peptides; cytostatic drugs; drug delivery; peptide-drug conjugates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Gestin M., Dowaidar M., Langel Ü. Uptake mechanism of cell-penetrating peptides. Adv. Exp. Med. Biol. 2017;1030:255–264. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

