Simulated Gastric and Intestinal Fluid Electrolyte Solutions as an Environment for the Adsorption of Apple Polyphenols onto β-Glucan
- PMID: 36235220
- PMCID: PMC9570717
- DOI: 10.3390/molecules27196683
Simulated Gastric and Intestinal Fluid Electrolyte Solutions as an Environment for the Adsorption of Apple Polyphenols onto β-Glucan
Abstract
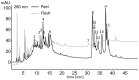
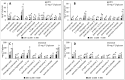
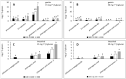
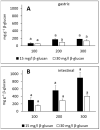
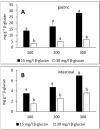
Interactions with dietary fibers in the gastrointestinal tract might affect the potential bioactivities of phenolic compounds. In this study, the interactions between apple phenolic compounds and β-glucan (a dietary fiber) were studied by studying the adsorption process in simulated gastric and intestinal fluid electrolyte solutions. Phenolic compounds were extracted from apples, adsorbed onto β-glucan (2 h, 37 °C, in gastric or intestinal fluid electrolyte solutions), and determined using high performance liquid chromatography. Phenolic compounds (flavan-3-ols, flavonols, phenolic acids, and dihydrochalcone) were stable in the gastric fluid (pH 3). In the intestinal fluid (pH 7), flavan-3-ols were not found and chlorogenic acid isomerized. Polyphenols from the apple peel (up to 182 and 897 mg g-1) and flesh (up to 28 and 7 mg g-1) were adsorbed onto β-glucan in the gastric and intestinal fluids, respectively. The adsorption was affected by the initial concentration of the polyphenols and β-glucan and by the environment (either gastric or intestinal fluid electrolyte solution). By increasing the initial polyphenol amount, the quantity of adsorbed polyphenols increased. Increasing the amount of β-glucan decreased the amount adsorbed. The results can be helpful in explaining the fate of phenolic compounds in the gastrointestinal tract.
Keywords: adsorption capacity; dietary fiber; gastrointestinal tract; phenolic compounds.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Interactions of polyphenols from traditional apple varieties 'Bobovac', 'Ljepocvjetka' and 'Crvenka' with β-Glucan during in vitro simulated digestion.Food Chem. 2021 Nov 30;363:130283. doi: 10.1016/j.foodchem.2021.130283. Epub 2021 Jun 4. Food Chem. 2021. PMID: 34120042
-
Polyphenols of Traditional Apple Varieties in Interaction with Barley β-Glucan: A Study of the Adsorption Process.Foods. 2020 Sep 11;9(9):1278. doi: 10.3390/foods9091278. Foods. 2020. PMID: 32933005 Free PMC article.
-
The Effect of β-Glucan on the Release and Antiradical Activity of Phenolic Compounds from Apples in Simulated Digestion.Molecules. 2025 Jan 14;30(2):301. doi: 10.3390/molecules30020301. Molecules. 2025. PMID: 39860171 Free PMC article.
-
The Behavior of Phenolic Compounds from Apples during Simulated Gastrointestinal Digestion with Focus on Chlorogenic Acid.Foods. 2024 Feb 24;13(5):693. doi: 10.3390/foods13050693. Foods. 2024. PMID: 38472806 Free PMC article.
-
Phenolic Compounds from Apples: From Natural Fruits to the Beneficial Effects in the Digestive System.Molecules. 2024 Jan 23;29(3):568. doi: 10.3390/molecules29030568. Molecules. 2024. PMID: 38338313 Free PMC article. Review.
Cited by
-
Pharmaceutical Insights Into Ammi and Parsley: Evaluating Antioxidant Activity, Total Phenolic Content, and Kidney Stone Disintegration Properties.Adv Pharmacol Pharm Sci. 2025 Feb 20;2025:5522905. doi: 10.1155/adpp/5522905. eCollection 2025. Adv Pharmacol Pharm Sci. 2025. PMID: 40018327 Free PMC article.
-
A Review on Optical Biosensors for Monitoring of Uric Acid and Blood Glucose Using Portable POCT Devices: Status, Challenges, and Future Horizons.Biosensors (Basel). 2025 Mar 31;15(4):222. doi: 10.3390/bios15040222. Biosensors (Basel). 2025. PMID: 40277536 Free PMC article. Review.
-
In Vitro Gastrointestinal Digestion of Various Sweet Potato Leaves: Polyphenol Profiles, Bioaccessibility and Bioavailability Elucidation.Antioxidants (Basel). 2024 Apr 26;13(5):520. doi: 10.3390/antiox13050520. Antioxidants (Basel). 2024. PMID: 38790625 Free PMC article.
-
Engineering Enhanced Antimicrobial Properties in α-Conotoxin RgIA through D-Type Amino Acid Substitution and Incorporation of Lysine and Leucine Residues.Molecules. 2024 Mar 6;29(5):1181. doi: 10.3390/molecules29051181. Molecules. 2024. PMID: 38474693 Free PMC article.
-
Synthesis of Cyclic Hexapeptides via the Hydrazide Method and Evaluation of Their Antibacterial Activities.Molecules. 2025 Jun 3;30(11):2444. doi: 10.3390/molecules30112444. Molecules. 2025. PMID: 40509331 Free PMC article.
References
-
- Lingua M.S., Theumer M.G., Kruzynski P., Wunderlin D.A., Baroni M.V. Bioaccessibility of polyphenols and antioxidant properties of the white grape by simulated digestion and Caco-2 cell assays: Comparative study with its winemaking product. Food Res. Int. 2019;122:496–505. doi: 10.1016/j.foodres.2019.05.022. - DOI - PubMed
-
- Quatrin A., Rampelotto C., Pauletto R., Maurer L.H., Nichelle S.M., Klein B., Fritzsche Rodrigues R., Maróstica Junior M.R., de Souza Fonseca B., Ragagnin de Menezes C., et al. Bioaccessibility and catabolism of phenolic compounds from jaboticaba (Myrciaria trunciflora) fruit peel during in vitro gastrointestinal digestion and colonic fermentation. J. Funct. Food. 2020;65:103714. doi: 10.1016/j.jff.2019.103714. - DOI
-
- Bouayed J., Deußer H., Hoffmann L., Bohn T. Bioaccessible and dialyzable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012;131:1466–1472. doi: 10.1016/j.foodchem.2011.10.030. - DOI
-
- Dias R., Pereira C.B., Pérez-Gregorio R., Mateus N., Freitas V. Recent advances on dietary polyphenol’s potential roles in Celiac Disease. Trends Food Sci. Technol. 2021;107:213–225. doi: 10.1016/j.tifs.2020.10.033. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

