Application of Biobased Solvents in Asymmetric Catalysis
- PMID: 36235236
- PMCID: PMC9570574
- DOI: 10.3390/molecules27196701
Application of Biobased Solvents in Asymmetric Catalysis
Abstract
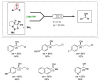
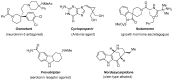
The necessity of more sustainable conditions that follow the twelve principles of Green Chemistry have pushed researchers to the development of novel reagents, catalysts and solvents for greener asymmetric methodologies. Solvents are in general a fundamental part for developing organic processes, as well as for the separation and purification of the reaction products. By this reason, in the last years, the application of the so-called green solvents has emerged as a useful alternative to the classical organic solvents. These solvents must present some properties, such as a low vapor pressure and toxicity, high boiling point and biodegradability, and must be obtained from renewable sources. In the present revision, the recent application of these biobased solvents in the synthesis of optically active compounds employing different catalytic methodologies, including biocatalysis, organocatalysis and metal catalysis, will be analyzed to provide a novel tool for carrying out more ecofriendly organic processes.
Keywords: biobased solvents; biocatalysis; green chemistry; metal catalysis; organocatalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

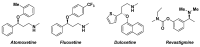

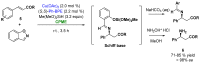
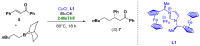
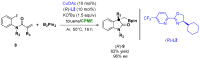

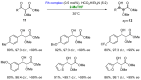


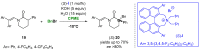
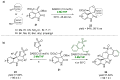

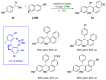
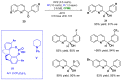
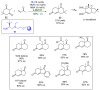
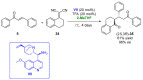
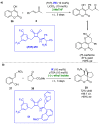
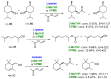
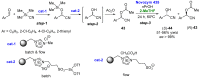
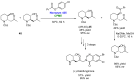
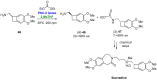

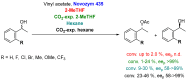
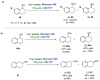
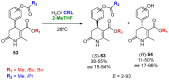
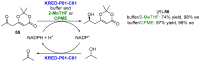
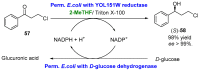
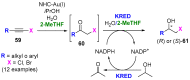
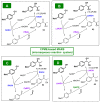
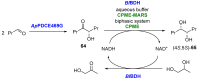
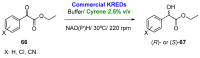
References
-
- Brown J.M., Davies S.G. Chemical asymmetric synthesis. Nature. 1989;342:631–636. doi: 10.1038/342631a0. - DOI
-
- Anil V., Karnik M.H. Stereochemistry, a Three-Dimensional Insight. 1st ed. Elsevier; Amsterdam, The Netherlands: 2021. pp. 407–463.
-
- Curzons A.D., Constable D.J.C., Mortimer D.N., Cunningham V.L. So you think your process is green, how do you know?—Using principles of sustainability to determine what is green–a corporate perspective. Green Chem. 2001;3:1–6. doi: 10.1039/b007871i. - DOI
-
- Jiménez-González C., Curzons A.D., Constable D.J.C., Cunningham V.L. Expanding GSK’s Solvent Selection Guide—Application of life cycle assessment to enhance solvent selections. Clean Technol. Environ. Policy. 2005;7:42–50. doi: 10.1007/s10098-004-0245-z. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

