Diaza-1,3-butadienes as Useful Intermediate in Heterocycles Synthesis
- PMID: 36235245
- PMCID: PMC9573662
- DOI: 10.3390/molecules27196708
Diaza-1,3-butadienes as Useful Intermediate in Heterocycles Synthesis
Abstract
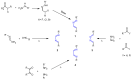
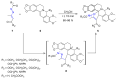
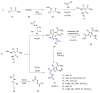
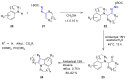
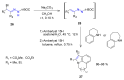
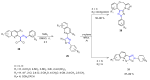
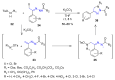
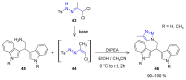
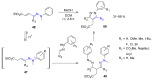
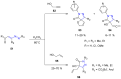
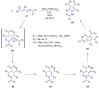
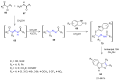
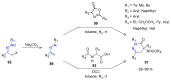
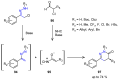
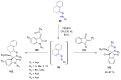
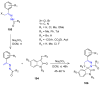
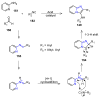
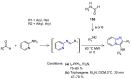
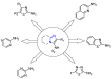
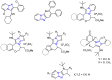
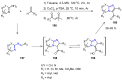
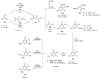
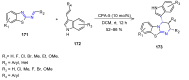
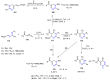
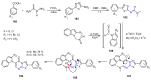
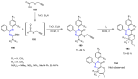
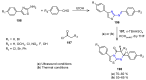
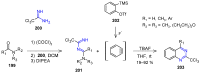
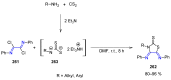
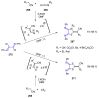
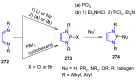
Many heterocyclic compounds can be synthetized using diaza-1,3-butadienes (DADs) as key structural precursors. Isolated and in situ diaza-1,3-butadienes, produced from their respective precursors (typically imines and hydrazones) under a variety of conditions, can both react with a wide range of substrates in many kinds of reactions. Most of these reactions discussed here include nucleophilic additions, Michael-type reactions, cycloadditions, Diels-Alder, inverse electron demand Diels-Alder, and aza-Diels-Alder reactions. This review focuses on the reports during the last 10 years employing 1,2-diaza-, 1,3-diaza-, 2,3-diaza-, and 1,4-diaza-1,3-butadienes as intermediates to synthesize heterocycles such as indole, pyrazole, 1,2,3-triazole, imidazoline, pyrimidinone, pyrazoline, -lactam, and imidazolidine, among others. Fused heterocycles, such as quinazoline, isoquinoline, and dihydroquinoxaline derivatives, are also included in the review.
Keywords: diaza-1,3-butadienes; heterocycles; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





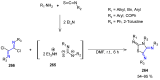
Similar articles
-
Recent Advances in Inverse-Electron-Demand Hetero-Diels-Alder Reactions of 1-Oxa-1,3-Butadienes.Top Curr Chem (Cham). 2016 Jun;374(3):24. doi: 10.1007/s41061-016-0026-2. Epub 2016 Apr 20. Top Curr Chem (Cham). 2016. PMID: 27573264 Free PMC article. Review.
-
Inverse Electron-Demand Aza-Diels-Alder Reaction of Cyclic Enamides with 1,2-Diaza-1,3-dienes in Situ Generated from α-Halogeno Hydrazones: Access to Fused Polycyclic Tetrahydropyridazine Derivatives.J Org Chem. 2021 Sep 3;86(17):11472-11481. doi: 10.1021/acs.joc.1c00993. Epub 2021 Aug 3. J Org Chem. 2021. PMID: 34343003
-
Synthesis of isoquinoline derivatives by Diels-Alder reactions of 2(1H)-pyridones having an electron-withdrawing group with methoxy-1,3-butadienes.Chem Pharm Bull (Tokyo). 2000 Nov;48(11):1814-7. doi: 10.1248/cpb.48.1814. Chem Pharm Bull (Tokyo). 2000. PMID: 11086925
-
Aromatic Heterocycles as Productive Dienophiles in the Inverse Electron-Demand Diels-Alder Reactions of 1,3,5-Triazines.Acc Chem Res. 2020 Apr 21;53(4):773-781. doi: 10.1021/acs.accounts.9b00604. Epub 2020 Mar 31. Acc Chem Res. 2020. PMID: 32227911
-
Inverse Electron Demand Diels-Alder Reactions of Heterocyclic Azadienes, 1-Aza-1,3-Butadienes, Cyclopropenone Ketals, and Related Systems. A Retrospective.J Org Chem. 2019 Aug 2;84(15):9397-9445. doi: 10.1021/acs.joc.9b00834. Epub 2019 May 23. J Org Chem. 2019. PMID: 31062977 Free PMC article. Review.
Cited by
-
Editorial: Heterodienes in organic synthesis.Front Chem. 2024 Apr 8;12:1403024. doi: 10.3389/fchem.2024.1403024. eCollection 2024. Front Chem. 2024. PMID: 38650672 Free PMC article. No abstract available.
References
-
- Belskaya N.P., Eliseeva A.I., Bakulev V.A. Hydrazones as substrates for cycloaddition reactions. Russ. Chem. Rev. 2015;84:1226–1257. doi: 10.1070/RCR4463. - DOI
-
- Zelenin K.N., Bezhan I.P., Matveeva Z.M. Synthesis of nitrogen heterocycles by (4+2)π-cycloaddition from nitrogen-containing heterodienes (“azadiene synthesis”) Chem. Heterocycl. Compd. 1972;8:525–534. doi: 10.1007/BF00488138. - DOI
-
- Sharma P., Kumar R., Bhargava G. Recent development in the synthesis of pyrrolin-4-ones/pyrrolin-3-ones. J. Heterocycl. Chem. 2020;57:4115–4135. doi: 10.1002/jhet.4143. - DOI
-
- Moskal J., Bronowski J., Rogowski A. Conjugated Schiff bases, XIII. sterically congested 1,4-diazabutadienes as dipolar reagents in 1,3-cycloaddition. Mon. Chem. Chem. Mon. 1981;112:1405–1416. doi: 10.1007/BF00900006. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

