Dextran Fluorescent Probes Containing Sulfadiazine and Rhodamine B Groups
- PMID: 36235281
- PMCID: PMC9571416
- DOI: 10.3390/molecules27196747
Dextran Fluorescent Probes Containing Sulfadiazine and Rhodamine B Groups
Abstract
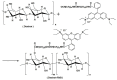
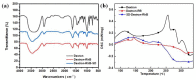
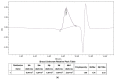
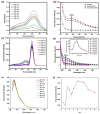
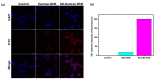
Fluorescent imaging has been expanded, as a non-invasive diagnostic modality for cancers, in recent years. Fluorescent probes in the near-infrared window can provide high sensitivity, resolution, and signal-to-noise ratio, without the use of ionizing radiation. Some fluorescent compounds with low molecular weight, such as rhodamine B (RhB) and indocyanine green (ICG), have been used in fluorescent imaging to improve imaging contrast and sensitivity; however, since these probes are excreted from the body quickly, they possess significant restrictions for imaging. To find a potential solution to this, this work investigated the synthesis and properties of novel macromolecular fluorescent compounds. Herein, water-soluble dextran fluorescent compounds (SD-Dextran-RhB) were prepared by the attachment of RhB and sulfadiazine (SD) derivatives to dextran carrier. These fluorescent compounds were then characterized through IR, 1H NMR, 13C NMR, UV, GPC, and other methods. Assays of their cellular uptake and cell cytotoxicity and fluorescent imaging were also performed. Through this study, it was found that SD-Dextran-RhB is sensitive to acidic conditions and possesses low cell cytotoxicities compared to normal 293 cells and HepG2 and HeLa tumor cells. Moreover, SD-Dextran-RhB demonstrated good fluorescent imaging in HepG2 and HeLa cells. Therefore, SD-Dextran-RhB is suitable to be potentially applied as a probe in the fluorescent imaging of tumors.
Keywords: dextran; fluorescent imaging; fluorescent probe; rhodamine B; sulfadiazine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Zhang Y.B., Sun L., Yan Q., Qiu X.Y., Cheng Y.T., Wang B.L., Tan X.P., Fang M.X., Luck R.L., Liu H.Y. Near-infrared fluorescent probe based on cyanine scaffold for sensitive detection of uranyl ions in living cells and water samples. Microchem. J. 2022;180:107619. doi: 10.1016/j.microc.2022.107619. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

