Enabling Trade in Gene-Edited Produce in Asia and Australasia: The Developing Regulatory Landscape and Future Perspectives
- PMID: 36235403
- PMCID: PMC9571430
- DOI: 10.3390/plants11192538
Enabling Trade in Gene-Edited Produce in Asia and Australasia: The Developing Regulatory Landscape and Future Perspectives
Abstract
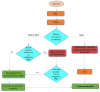
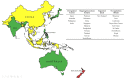
Genome- or gene-editing (abbreviated here as 'GEd') presents great opportunities for crop improvement. This is especially so for the countries in the Asia-Pacific region, which is home to more than half of the world's growing population. A brief description of the science of gene-editing is provided with examples of GEd products. For the benefits of GEd technologies to be realized, international policy and regulatory environments must be clarified, otherwise non-tariff trade barriers will result. The status of regulations that relate to GEd crop products in Asian countries and Australasia are described, together with relevant definitions and responsible regulatory bodies. The regulatory landscape is changing rapidly: in some countries, the regulations are clear, in others they are developing, and some countries have yet to develop appropriate policies. There is clearly a need for the harmonization or alignment of GEd regulations in the region: this will promote the path-to-market and enable the benefits of GEd technologies to reach the end-users.
Keywords: Asia; Asia-Pacific; Australasia; Cas9; GEd; biosafety; crops; gene editing; genome editing; harmonization; path-to-market; regulations; science diplomacy; trade.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- AgbioInvestor . A Study on Behalf of Crop Life International: Cost and Time Required for the Discovery, Development and Authorisation of a New Plant Biotechnology-Derived Genetic Trait. 2022. [(accessed on 17 July 2022)]. Time and cost to develop a new GM trait; pp. 1–45. Available online: www.agbioinvestor.com.
Publication types
Grants and funding
- [BB/J004588/1] [BB/P013511/1]/Biotechnology and Biological Sciences Research Council (BBSRC) Institute Strategic Programmes GRO; GEN
- 4-FA4N7WL/Department of Agriculture, Forestry and Fisheries(formerly DAWE), Government of Australia, Project Assisting Small Exporters (PASE), Building Capacity for Small Exporters to Exploit' new breeding technologies'
LinkOut - more resources
Full Text Sources

