Bioactivity and Therapeutic Potential of Kaempferol and Quercetin: New Insights for Plant and Human Health
- PMID: 36235488
- PMCID: PMC9571405
- DOI: 10.3390/plants11192623
Bioactivity and Therapeutic Potential of Kaempferol and Quercetin: New Insights for Plant and Human Health
Abstract
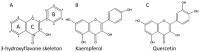
Plant secondary metabolites, especially flavonoids, are major metabolites widely found in plants that play several key roles in plant defence and signalling in response to stress conditions. The most studied among these flavonoids are kaempferol and quercetin due to their anti-oxidative potential and their key roles in the defence system, making them more critical for plant adaptation in stress environments. Kaempferol and quercetin in plants have great therapeutic potential for human health. Despite being well-studied, some of their functional aspects regarding plants and human health need further evaluation. This review summarizes the emerging potential of kaempferol and quercetin in terms of antimicrobial activity, bioavailability and bioactivity in the human body as well as in the regulation of plant defence in response to stresses and as a signalling molecule in terms of hormonal modulation under stress conditions. We also evaluated the safe use of both metabolites in the pharmaceutical industry.
Keywords: antioxidant; bioactivity; bioavailability; kaempferol; quercetin; therapeutic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans.Nutrients. 2019 Sep 25;11(10):2288. doi: 10.3390/nu11102288. Nutrients. 2019. PMID: 31557798 Free PMC article. Review.
-
Plant Flavonoids in Mediterranean Species: A Focus on Flavonols as Protective Metabolites under Climate Stress.Plants (Basel). 2022 Jan 10;11(2):172. doi: 10.3390/plants11020172. Plants (Basel). 2022. PMID: 35050060 Free PMC article. Review.
-
Probing the Potential Mechanism of Quercetin and Kaempferol against Heat Stress-Induced Sertoli Cell Injury: Through Integrating Network Pharmacology and Experimental Validation.Int J Mol Sci. 2022 Sep 22;23(19):11163. doi: 10.3390/ijms231911163. Int J Mol Sci. 2022. PMID: 36232461 Free PMC article.
-
Assessing Anticancer Potential of Blueberry Flavonoids, Quercetin, Kaempferol, and Gentisic Acid, Through Oxidative Stress and Apoptosis Parameters on HCT-116 Cells.J Med Food. 2019 Nov;22(11):1118-1126. doi: 10.1089/jmf.2019.0098. Epub 2019 Jun 26. J Med Food. 2019. PMID: 31241392
-
Quercetin and Related Analogs as Therapeutics to Promote Tissue Repair.Bioengineering (Basel). 2023 Sep 25;10(10):1127. doi: 10.3390/bioengineering10101127. Bioengineering (Basel). 2023. PMID: 37892857 Free PMC article. Review.
Cited by
-
Chemical Composition and Antioxidant Activity of Six Allium Extracts Using Protein-Based Biomimetic Methods.Antioxidants (Basel). 2024 Sep 29;13(10):1182. doi: 10.3390/antiox13101182. Antioxidants (Basel). 2024. PMID: 39456436 Free PMC article.
-
Cytocompatibility, Antimicrobial and Antioxidant Activity of a Mucoadhesive Biopolymeric Hydrogel Embedding Selenium Nanoparticles Phytosynthesized by Sea Buckthorn Leaf Extract.Pharmaceuticals (Basel). 2023 Dec 22;17(1):23. doi: 10.3390/ph17010023. Pharmaceuticals (Basel). 2023. PMID: 38256857 Free PMC article.
-
Integrated transcriptomic and metabolomic analyses elucidate the mechanism of flavonoid biosynthesis in the regulation of mulberry seed germination under salt stress.BMC Plant Biol. 2024 Feb 21;24(1):132. doi: 10.1186/s12870-024-04804-3. BMC Plant Biol. 2024. PMID: 38383312 Free PMC article.
-
Phytochemical Profiling, Antioxidant and Antimicrobial Potentials of Ethanol and Ethyl Acetate Extracts of Chamaenerion latifolium L.Pharmaceuticals (Basel). 2024 Jul 27;17(8):996. doi: 10.3390/ph17080996. Pharmaceuticals (Basel). 2024. PMID: 39204101 Free PMC article.
-
Synthesis and Characterization of New Pyrano[2,3-c]pyrazole Derivatives as 3-Hydroxyflavone Analogues.Molecules. 2023 Sep 13;28(18):6599. doi: 10.3390/molecules28186599. Molecules. 2023. PMID: 37764375 Free PMC article.
References
-
- Jan R., Asaf S., Numan M., Kim K.-M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy. 2021;11:968. doi: 10.3390/agronomy11050968. - DOI
-
- Saini N., Gahlawat S., Lather V. Plant Biotechnology: Recent Advancements and Developments. Springer; Berlin/Heidelberg, Germany: 2017. Flavonoids: A nutraceutical and its role as anti-inflammatory and anticancer agent; pp. 255–270.
Publication types
LinkOut - more resources
Full Text Sources

