Photosynthetic Apparatus of Hydrocharis morsus-ranae in Different Solar Lighting
- PMID: 36235524
- PMCID: PMC9571613
- DOI: 10.3390/plants11192658
Photosynthetic Apparatus of Hydrocharis morsus-ranae in Different Solar Lighting
Abstract
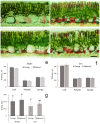
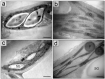
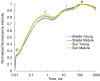
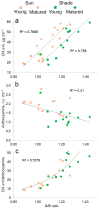
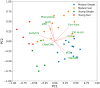
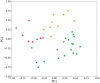
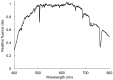
Hydrocharis morsus-ranae is a free-floating species growing in lakes and slow-flowing rivers near the shore in Europe and Western Asia, and as an invasive plant in the USA and Canada. Light-requiring plants of this species can also grow in the shade, up to about 30% of full sunlight. In this paper we present the data about the photosynthetic apparatus of sunny and shady H. morsus-ranae plants grown in the sun and in the shade in nature. Methods of light and transmission electron microscopy, biochemistry, chlorophyll fluorescence induction as well as the principal component analysis were used. It was found that leaves of plants growing in shade differed from those in the sun with such traits as thickness of a blade, palisade and spongy parenchyma, ultrastructure of chloroplasts, and quantum efficiency of photosynthetic electron transport, the content of chlorophylls and carotenoids, anthocyanins and phenilpropanoids. By these traits, H. morsus-ranae shady plants are similar with shade-bearing plants that indicates their adaptation to light intensity lowering. The ordination plots (PCA) suggested a clear structural and functional shift of plants growing in different lighting showing relationship to light changes in the natural environment. Thus, our results displayed the high phenotypic plasticity of the H. morsus-ranae photosynthetic apparatus, which ensures its acclimation to changing light environment and wide distribution of this species.
Keywords: acclimation; anthocyanin; chlorophyll induction; chloroplast; granum; pigment complex; plasticity; principal component analysis; shade; sunlight.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Cook C.D.K., Luond R. A revision of the genus Hydrocharis (Hydrocharitaceae) Aquat. Bot. 1982;14:177–204. doi: 10.1016/0304-3770(82)90097-3. - DOI
-
- Scribailo R.W., Posluszny U. The reproductive biology of Hydrocharis morsus-ranae L. Floral biology. Canad. J. Bot. 1984;62:2779–2787. doi: 10.1139/b84-370. - DOI
-
- Catling P.M., Miltrow G., Haber E., Posluszny U., Charlton W.A. The biology of Canadian weeds Hydrocharis morsus-ranae L. Can. J. Plant Sci. 2003;83:1001–1016. doi: 10.4141/P02-033. - DOI
-
- Toma C. Reproduction of Hydrocharis morsus-ranae taxa in an oxbow lake of the River Vistula. Limnol. Rev. 2013;3:171–179. doi: 10.2478/limre-2013-0019. - DOI
-
- Zhu B., Ottaviani C.C., Naddafi R., Dai Z., Du D. Invasive European frogbit (Hydrocharis morsus-ranae L.) in North America: An updated review 2003–2016. J. Plant Ecol. 2018;11:17–25. doi: 10.1093/jpe/rtx031. - DOI
LinkOut - more resources
Full Text Sources

