Formulation and Evaluation of Fenbendazole Extended-Release Extrudes Processed by Hot-Melt Extrusion
- PMID: 36236135
- PMCID: PMC9573241
- DOI: 10.3390/polym14194188
Formulation and Evaluation of Fenbendazole Extended-Release Extrudes Processed by Hot-Melt Extrusion
Abstract
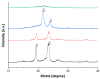
This study aimed to demonstrate the feasibility of hot-melt extrusion in the development of extended-release formulations of Fenbendazole (Fen) dispersed in PEO/PCL blend-based matrices. Their thermal, physical, chemical and viscosity properties were assessed by differential scanning calorimetry, thermogravimetric analysis/derivative thermogravimetry, Fourier transform infrared spectroscopy, X-ray diffraction spectroscopy, and melt flow index. Drug dispersion was analyzed by scanning electron microscopy with electron dispersive X-ray spectroscopy, and drug release was evaluated by ultraviolet-visible spectroscopy. A thermal analysis indicated the conversion of the drug to its amorphous state. FTIR analysis endorsed the thermal studies pointing to a decrease in the drug's crystallinity with the establishment of intermolecular interactions. XRD analysis confirmed the amorphous nature of Fen. MFI test revealed that PCL acts as a plasticizer when melt-processed with PEO. SEM images displayed irregular surfaces with voids and pores, while EDX spectra demonstrated a homogeneous drug distribution throughout the polymeric carrier. Dissolution testing revealed that PCL retards the drug release proportionally to the content of such polymer incorporated. These melt-extruded matrices showed that the drug release rate in a PEO/PCL blend can easily be tailored by altering the ratio of PCL to address the issues related to the multiple-dosing regimen of Fen in ruminants.
Keywords: extended-release; fenbendazole; hot-melt extrusion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Page S.W. Small Animal Clinical Pharmacology. 2nd ed. Elsevier Ltd.; Amsterdam, The Netherlands: 2008. Antiparasitic drugs. - DOI
-
- Grehan L., Killion J.A., Devine D.M., Kenny E.K., Devery S., Higginbotham C.L., Geever L.M. The Development of Hot Melt Extruded Biocompatible Controlled Release Drug Delivery Devices. Int. J. Polym. Mater. Polym. Biomater. 2014;63:476–485. doi: 10.1080/00914037.2013.854218. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

