Developmental heterogeneity of embryonic neuroendocrine chromaffin cells and their maturation dynamics
- PMID: 36237181
- PMCID: PMC9553123
- DOI: 10.3389/fendo.2022.1020000
Developmental heterogeneity of embryonic neuroendocrine chromaffin cells and their maturation dynamics
Abstract
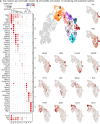
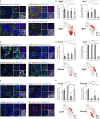
During embryonic development, nerve-associated Schwann cell precursors (SCPs) give rise to chromaffin cells of the adrenal gland via the "bridge" transient stage, according to recent functional experiments and single cell data from humans and mice. However, currently existing data do not resolve the finest heterogeneity of developing chromaffin populations. Here we took advantage of deep SmartSeq2 transcriptomic sequencing to expand our collection of individual cells from the developing murine sympatho-adrenal anlage and uncover the microheterogeneity of embryonic chromaffin cells and their corresponding developmental paths. We discovered that SCPs on the splachnic nerve show a high degree of microheterogeneity corresponding to early biases towards either Schwann or chromaffin terminal fates. Furthermore, we found that a post-"bridge" population of developing chromaffin cells gives rise to persisting oxygen-sensing chromaffin cells and the two terminal populations (adrenergic and noradrenergic) via diverging differentiation paths. Taken together, we provide a thorough identification of novel markers of adrenergic and noradrenergic populations in developing adrenal glands and report novel differentiation paths leading to them.
Keywords: SmartSeq2; adrenal medulla; chromaffin cell; microheterogeneity; single-cell transcriptomics; sympathoadrenal development.
Copyright © 2022 Akkuratova, Faure, Kameneva, Kastriti and Adameyko.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relatthat could be construed as a potential conflict of interest.
Figures





Similar articles
-
SNAP-25 regulation during adrenal gland development: comparison with differentiation markers and other SNAREs.J Comp Neurol. 2000 Jun 12;421(4):533-42. J Comp Neurol. 2000. PMID: 10842212
-
Human fetal chromaffin cells: a potential tool for cell pain therapy.Exp Neurol. 2007 Jun;205(2):525-35. doi: 10.1016/j.expneurol.2007.03.020. Epub 2007 Mar 27. Exp Neurol. 2007. PMID: 17466976
-
Transcription factor AP-2β regulates the neurotransmitter phenotype and maturation of chromaffin cells.Mol Cell Neurosci. 2011 Jan;46(1):245-51. doi: 10.1016/j.mcn.2010.09.007. Epub 2010 Sep 27. Mol Cell Neurosci. 2011. PMID: 20875861 Free PMC article.
-
Generation of neuroendocrine chromaffin cells from sympathoadrenal progenitors: beyond the glucocorticoid hypothesis.Ann N Y Acad Sci. 2002 Oct;971:554-9. doi: 10.1111/j.1749-6632.2002.tb04526.x. Ann N Y Acad Sci. 2002. PMID: 12438182 Review.
-
Stem cells, evolutionary aspects and pathology of the adrenal medulla: A new developmental paradigm.Mol Cell Endocrinol. 2020 Dec 1;518:110998. doi: 10.1016/j.mce.2020.110998. Epub 2020 Aug 18. Mol Cell Endocrinol. 2020. PMID: 32818585 Review.
Cited by
-
Hypoxia-Inducible Factor 2α: at the Interface between Oxygen Sensing Systems in Physiology and Pathology.Physiology (Bethesda). 2025 Sep 1;40(5):0. doi: 10.1152/physiol.00043.2024. Epub 2025 Feb 13. Physiology (Bethesda). 2025. PMID: 39946558 Review.
-
Comparative Single-Cell Transcriptomics of Human Neuroblastoma and Preclinical Models Reveals Conservation of an Adrenergic Cell State.Cancer Res. 2025 Mar 14;85(6):1015-1034. doi: 10.1158/0008-5472.CAN-24-1507. Cancer Res. 2025. PMID: 39808065 Free PMC article.
-
Hif-2α programs oxygen chemosensitivity in chromaffin cells.J Clin Invest. 2024 Aug 6;134(18):e174661. doi: 10.1172/JCI174661. J Clin Invest. 2024. PMID: 39106106 Free PMC article.
References
-
- Zuckerkandl E. About sympathetic paraganglions in the retroperitoneal space of man. (Über nebenorgane des sympathacus im retroperitonealraum des menschen). Verh Anat Ges (1901) 15:95–107.
-
- Kohn A. Die Paraganglien. Archiv f mikrosk Anat (1903) 62:263–365. doi: 10.1007/BF02985550 - DOI
-
- Coupland RE. The natural history of the chromaffin cell. Arch Histol Cytol (1965) 52(Suppl):331–41 Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1270777/. - PubMed
-
- Böck P. The paraganglia Handbuch der mikroskopischen anatomie des menschen. Springer Sci Business Med pp1 (1982) VI(Part 8.):27–30.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

