Self-supervised learning mechanism for identification of eyelid malignant melanoma in pathologic slides with limited annotation
- PMID: 36237543
- PMCID: PMC9550873
- DOI: 10.3389/fmed.2022.976467
Self-supervised learning mechanism for identification of eyelid malignant melanoma in pathologic slides with limited annotation
Abstract
Purpose: The lack of finely annotated pathologic data has limited the application of deep learning systems (DLS) to the automated interpretation of pathologic slides. Therefore, this study develops a robust self-supervised learning (SSL) pathology diagnostic system to automatically detect malignant melanoma (MM) in the eyelid with limited annotation.
Design: Development of a self-supervised diagnosis pipeline based on a public dataset, then refined and tested on a private, real-world clinical dataset.
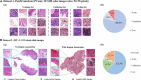
Subjects: A. Patchcamelyon (PCam)-a publicly accessible dataset for the classification task of patch-level histopathologic images. B. The Second Affiliated Hospital, Zhejiang University School of Medicine (ZJU-2) dataset - 524,307 patches (small sections cut from pathologic slide images) from 192 H&E-stained whole-slide-images (WSIs); only 72 WSIs were labeled by pathologists.
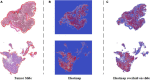
Methods: Patchcamelyon was used to select a convolutional neural network (CNN) as the backbone for our SSL-based model. This model was further developed in the ZJU-2 dataset for patch-level classification with both labeled and unlabeled images to test its diagnosis ability. Then the algorithm retrieved information based on patch-level prediction to generate WSI-level classification results using random forest. A heatmap was computed for visualizing the decision-making process.
Main outcome measures: The area under the receiver operating characteristic curve (AUC), accuracy, sensitivity, and specificity were used to evaluate the performance of the algorithm in identifying MM.
Results: ResNet50 was selected as the backbone of the SSL-based model using the PCam dataset. This algorithm then achieved an AUC of 0.981 with an accuracy, sensitivity, and specificity of 90.9, 85.2, and 96.3% for the patch-level classification of the ZJU-2 dataset. For WSI-level diagnosis, the AUC, accuracy, sensitivity, and specificity were 0.974, 93.8%, 75.0%, and 100%, separately. For every WSI, a heatmap was generated based on the malignancy probability.
Conclusion: Our diagnostic system, which is based on SSL and trained with a dataset of limited annotation, can automatically identify MM in pathologic slides and highlight MM areas in WSIs by a probabilistic heatmap. In addition, this labor-saving and cost-efficient model has the potential to be refined to help diagnose other ophthalmic and non-ophthalmic malignancies.
Keywords: artificial intelligence - assisted bioinformatic analysis; melanoma; pathology; self-supervised deep learning; tumor diagnosis.
Copyright © 2022 Wang, Jiang, Shao, Liu, Gu, Ge, Jia, Wang and Ye.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Cummins DL, Cummins JM, Pantle H, Silverman MA, Leonard AL, Chanmugam A. Cutaneous malignant melanoma. Mayo Clin Proc. (2006) 81:500–7. - PubMed
-
- Malhotra R, Chen C, Huilgol SC, Hill DC, Selva D. Mapped serial excision for periocular lentigo maligna and lentigo maligna melanoma. Ophthalmology. (2003) 110:2011–8. - PubMed
-
- Catalyürek U, Beynon MD, Chang C, Kurc T, Sussman A, Saltz J. The virtual microscope. IEEE Trans Inf Technol Biomed. (2003) 7:230–48. - PubMed
LinkOut - more resources
Full Text Sources

