Dinickel Catalyzed Vinylidene-Alkene Cyclization Reactions
- PMID: 36237605
- PMCID: PMC9552772
- DOI: 10.1021/acscatal.1c03350
Dinickel Catalyzed Vinylidene-Alkene Cyclization Reactions
Abstract
A dinickel catalyst promotes reductive cyclization reactions of 1,1-dichloroalkenes containing pendant olefins. The reactions can be conducted with a Zn reductant or electrocatalytically using a carbon working electrode. Mechanistic studies are consistent with the intermediacy of a Ni2(vinylidene) species, which adds to the alkene and generates a metallacyclic intermediate. β-Hydride elimination followed by C-H reductive elimination forms the cyclization product. The proposed dinickel metallacycle is structurally characterized and its stoichiometric conversion to product is demonstrated. Spin polarized, unrestricted DFT calculations are used to further examine the cyclization mechanism. These computational models reveal that both nickel centers function cooperatively to mediate the key oxidative addition, migratory insertion, β-hydride elimination, and reductive elimination steps.
Keywords: cyclization; electrocatalysis; metal–metal bonds; nickel; vinylidene.
Figures







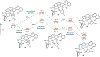
References
-
- Ojima I; Tzamarioudaki M; Li Z; Donovan RJ “Transition Metal-Catalyzed Carbocyclizations in Organic Synthesis” Chem. Rev 1996, 96, 635–662; - PubMed
- Aubert C; Buisine O; Malacria M “The Behavior of 1,n-Enynes in the Presence of Transition Metals” Chem. Rev 2002, 102, 813–834; - PubMed
- Lloyd-Jones GC “Mechanistic aspects of transition metal catalysed 1,6-diene and 1,6-enyne cycloisomerisation reactions” Org. Biomol. Chem 2003, 1, 215–236; - PubMed
- Echavarren AM; Nevado C “Non-stabilized transition metal carbenes as intermediates in intramolecular reactions of alkynes with alkenes” Chem. Soc. Rev 2004, 33, 431–436; - PubMed
- Zhang L; Sun J; Kozmin SA “Gold and Platinum Catalysis of Enyne Cycloisomerization” Adv. Synth. Catal 2006, 348, 2271–2296;
- Michelet V; Toullec PY; Genêt J-P “Cycloisomerization of 1,n-Enynes: Challenging Metal-Catalyzed Rearrangements and Mechanistic Insights” Angew. Chem., Int. Ed 2008, 47, 4268–4315. - PubMed
-
- Nuñez-Zarur F; Solans-Monfort X; Rodríguez-Santiago L; Sodupe M “Exo/endo Selectivity of the Ring-Closing Enyne Methathesis Catalyzed by Second Generation Ru-Based Catalysts. Influence of Reactant Substituents” ACS Catal. 2013, 3, 206–218;
- Lee OS; Kim KH; Kim J; Kwon K; Ok T; Ihee H; Lee H-Y; Sohn J-H “Correlation between Functionality Preference of Ru Carbenes and exo/endo Product Selectivity for Clarifying the Mechanism of Ring-Closing Enyne Metathesis” J. Org. Chem 2013, 78, 8242–8249. - PubMed
-
- Grigg R; Stevenson P; Worakun T “Rhodium (I) catalysed regiospecific cyclisation of 1,6-enynes to methylenecyclohex-2-enes” Tetrahedron 1988, 44, 4967–4972.
-
- Kim H; Lee C “ Cycloisomerization of Enynes via Rhodium Vinylidene-Mediated Catalysis” J. Am. Chem. Soc 2005, 127, 10180–10181. - PubMed
-
- Bruneau C; Dixneuf PH “Metal Vinylidenes and Allenylidenes in Catalysis: Applications in Anti-Markovnikov Additions to Terminal Alkynes and Alkene Metathesis” Angew. Chem., Int. Ed 2006, 45, 2176–2203; - PubMed
- Bruneau C; Dixneuf P Metal vinylidenes and allenylidenes in catalysis: from reactivity to applications in synthesis; John Wiley & Sons, 2008;
- Trost BM; McClory A “Metal Vinylidenes as Catalytic Species in Organic Reactions” Chem. Asian J 2008, 3, 164–194; - PMC - PubMed
- Roh SW; Choi K; Lee C “Transition Metal Vinylidene- and Allenylidene-Mediated Catalysis in Organic Synthesis” Chem. Rev 2019, 119, 4293–4356. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
