LI-RADS Treatment Response versus Modified RECIST for Diagnosing Viable Hepatocellular Carcinoma after Locoregional Therapy: A Systematic Review and Meta-Analysis of Comparative Studies
- PMID: 36237934
- PMCID: PMC9514432
- DOI: 10.3348/jksr.2021.0173
LI-RADS Treatment Response versus Modified RECIST for Diagnosing Viable Hepatocellular Carcinoma after Locoregional Therapy: A Systematic Review and Meta-Analysis of Comparative Studies
Abstract
Purpose: To systematically compare the performance of liver imaging reporting and data system treatment response (LR-TR) with the modified Response Evaluation Criteria in Solid Tumors (mRECIST) for diagnosing viable hepatocellular carcinoma (HCC) treated with locoregional therapy (LRT).
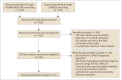
Materials and methods: Original studies of intra-individual comparisons between the diagnostic performance of LR-TR and mRECIST using dynamic contrast-enhanced CT or MRI were searched in MEDLINE and EMBASE, up to August 25, 2021. The reference standard for tumor viability was surgical pathology. The meta-analytic pooled sensitivity and specificity of the viable category using each criterion were calculated using a bivariate random-effects model and compared using bivariate meta-regression.
Results: For five eligible studies (430 patients with 631 treated observations), the pooled per-lesion sensitivities and specificities were 58% (95% confidence interval [CI], 45%-70%) and 93% (95% CI, 88%-96%) for the LR-TR viable category and 56% (95% CI, 42%-69%) and 86% (95% CI, 72%-94%) for the mRECIST viable category, respectively. The LR-TR viable category provided significantly higher pooled specificity (p < 0.01) than the mRECIST but comparable pooled sensitivity (p = 0.53).
Conclusion: The LR-TR algorithm demonstrated better specificity than mRECIST, without a significant difference in sensitivity for the diagnosis of pathologically viable HCC after LRT.
목적: 국소 치료 후 잔존 간세포암 진단을 위한 LI-RADS 치료 반응(liver imaging reporting and data system treatment response; 이하 LR-TR)과 modified Response Evaluation Criteria in Solid Tumors (이하 mRECIST) 기준의 진단능을 체계적으로 비교한다.
대상과 방법: MEDLINE과 EMBASE에서 역동적 조영증강 CT 또는 MRI를 이용하여 LR-TR과 mRECIST의 진단능을 개인 내 비교한 원저를 검색하였다. 생존 종양에 대한 참조 표준은 수술을 통한 병리 진단을 사용하였다. 각 기준의 생존 카테고리에 대한 메타분석적 통합 민감도와 특이도는 bivariate random-effects model을 통해 계산하였고 bivariate meta-regression을통해 비교하였다.
결과: 총 다섯 개의 포함된 연구들에서(430명 환자들 및 631개 치료된 병변들), LR-TR 생존 카테고리의 병변별 통합 민감도와 특이도는 58% (95% 신뢰구간, 45%–70%)와 93% (95% 신뢰구간, 88%–96%)이었으며 mRECIST 생존 카테고리는 56% (95% 신뢰구간, 42%–69%)와 86% (95% 신뢰구간, 72%–94%)이었다. LR-TR 생존 카테고리는 mRECIST에 비하여 유의하게 높은 특이도를 보였으나(p < 0.01) 민감도는 유사하였다(p = 0.53).
결론: LR-TR 알고리즘은 국소 치료 후 병리학적 잔존 간세포암의 진단에 대하여 민감도의 유의한 차이 없이 mRECIST보다 높은 특이도를 보였다.
Keywords: Hepatocellular Carcinoma; Meta-Analysis; Response Evaluation Criteria in Solid Tumors; Systematic Review; Treatment Outcome.
Copyrights © 2022 The Korean Society of Radiology.
Conflict of interest statement
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Figures

References
-
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. - PubMed
-
- Ho MH, Yu CY, Chung KP, Chen TW, Chu HC, Lin CK, et al. Locoregional therapy-induced tumor necrosis as a predictor of recurrence after liver transplant in patients with hepatocellular carcinoma. Ann Surg Oncol. 2011;18:3632–3639. - PubMed
-
- Allard MA, Sebagh M, Ruiz A, Guettier C, Paule B, Vibert E, et al. Does pathological response after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients with cirrhosis predict outcome after liver resection or transplantation? J Hepatol. 2015;63:83–92. - PubMed
-
- Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. - PubMed
LinkOut - more resources
Full Text Sources

