Whole-cell vaccine candidates induce a protective response against virulent Acinetobacter baumannii
- PMID: 36238282
- PMCID: PMC9553005
- DOI: 10.3389/fimmu.2022.941010
Whole-cell vaccine candidates induce a protective response against virulent Acinetobacter baumannii
Abstract
Acinetobacter baumannii causes multi-system diseases in both nosocomial settings and a pre-disposed general population. The bacterium is not only desiccation-resistant but also notoriously resistant to multiple antibiotics and drugs of last resort including carbapenem, colistin, and sulbactam. The World Health Organization has categorized carbapenem-resistant A. baumannii at the top of its critical pathogen list in a bid to direct urgent countermeasure development. Several early-stage vaccines have shown a range of efficacies in healthy mice, but no vaccine candidates have advanced into clinical trials. Herein, we report our findings that both an ionizing γ-radiation-inactivated and a non-ionizing ultraviolet C-inactivated whole-cell vaccine candidate protects neutropenic mice from pulmonary challenge with virulent AB5075, a particularly pathogenic isolate. In addition, we demonstrate that a humoral response is sufficient for this protection via the passive immunization of neutropenic mice.
Keywords: A. baumannii; MDP; UVC; humoral; protection; pulmonary; vaccine; whole-cell.
Copyright © 2022 Dollery, Zurawski, Bushnell, Tobin, Wiggins, MacLeod, Tasker, Alamneh, Abu-Taleb, Czintos, Su, Escatte, Meeks, Daly and Tobin.
Conflict of interest statement
SJD, RVB, JKT, TJW, DAM, NJPERT, and GJT are employees of Biological Mimetics, Inc. The commercial nature of Biological Mimetics, Inc. does not alter the belief in, or the ability to adhere to the policies of the journal regarding data or material sharing or wider ethical standards. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
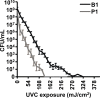
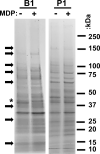

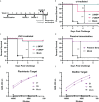
References
-
- WHO . Priority pathogens list for R&D of new antibiotics (2017). Available at: https://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-ba....
-
- CDC . Antibiotic resistance threats in the united states, 2019. Atlanta, GA: U.S. Department of Health and Human Services, CDC; (2019).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

