Dysregulated autoantibodies targeting vaso- and immunoregulatory receptors in Post COVID Syndrome correlate with symptom severity
- PMID: 36238301
- PMCID: PMC9552223
- DOI: 10.3389/fimmu.2022.981532
Dysregulated autoantibodies targeting vaso- and immunoregulatory receptors in Post COVID Syndrome correlate with symptom severity
Abstract
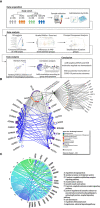
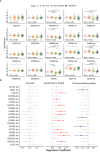
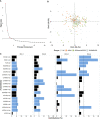
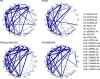
Most patients with Post COVID Syndrome (PCS) present with a plethora of symptoms without clear evidence of organ dysfunction. A subset of them fulfills diagnostic criteria of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Symptom severity of ME/CFS correlates with natural regulatory autoantibody (AAB) levels targeting several G-protein coupled receptors (GPCR). In this exploratory study, we analyzed serum AAB levels against vaso- and immunoregulatory receptors, mostly GPCRs, in 80 PCS patients following mild-to-moderate COVID-19, with 40 of them fulfilling diagnostic criteria of ME/CFS. Healthy seronegative (n=38) and asymptomatic post COVID-19 controls (n=40) were also included in the study as control groups. We found lower levels for various AABs in PCS compared to at least one control group, accompanied by alterations in the correlations among AABs. Classification using random forest indicated AABs targeting ADRB2, STAB1, and ADRA2A as the strongest classifiers (AABs stratifying patients according to disease outcomes) of post COVID-19 outcomes. Several AABs correlated with symptom severity in PCS groups. Remarkably, severity of fatigue and vasomotor symptoms were associated with ADRB2 AAB levels in PCS/ME/CFS patients. Our study identified dysregulation of AAB against various receptors involved in the autonomous nervous system (ANS), vaso-, and immunoregulation and their correlation with symptom severity, pointing to their role in the pathogenesis of PCS.
Keywords: COVID-19; Chronic Fatigue Syndrome; G-protein coupled receptor; ME/CFS,; autoantibodies; autonomic nervous system; post COVID syndrome; renin-angiotensin system.
Copyright © 2022 Sotzny, Filgueiras, Kedor, Freitag, Wittke, Bauer, Sepúlveda, Mathias da Fonseca, Baiocchi, Marques, Kim, Lange, Plaça, Luebber, Paulus, De Vito, Jurisica, Schulze-Forster, Paul, Bellmann-Strobl, Rust, Hoppmann, Shoenfeld, Riemekasten, Heidecke, Cabral-Marques and Scheibenbogen.
Conflict of interest statement
The authors declare that HH and KS-F are managing directors of CellTrend. CellTrend holds together with Charité a patent for the diagnostic use of AABs against ADRB2. CS has a consulting agreement with CellTrend. FP reports grants from the Guthy Jackson Charitable Foundation, during the conduct of the study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor MD declared a past co-authorship with the author YS.
Figures

References
-
- Kedor C, Freitag H, Meyer-Arndt L, Wittke K, Hanitsch LG, Zoller T, et al. . A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nature communications (2022) 13(1):5104. doi: 10.1038/s41467-022-32507-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

