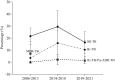
Temporal trend of drug-resistant tuberculosis among Thai children during 2006-2021
- PMID: 36238580
- PMCID: PMC9550601
- DOI: 10.1016/j.ijregi.2022.09.005
Temporal trend of drug-resistant tuberculosis among Thai children during 2006-2021
Abstract
Background: The prevalence of drug-resistant tuberculosis (DR-TB) in adults has stabilized in the past decade. Our study aimed to describe the prevalence of DR-TB in Thai children between 2006 and 2021.
Materials and methods: Children younger than 15 years old who had culture-confirmed Mycobacterium tuberculosis complex (MTB), positive PCR-MTB, or positive Xpert MTB/RIF were included in this cohort. Drug susceptibility testing (DST) was performed using phenotypic and/or genotypic methods. The prevalence of DR-TB was compared using the chi-square test.
Results: Among 163 confirmed TB cases (44% as pulmonary TB, 27% as extrapulmonary TB, and 29% with both), the median age (IQR) was 12.2 (7.3-14.2) years. DST was performed in 139 cases (85%), revealing prevalences of all DR-TB, isoniazid-resistant TB (Hr-TB), and rifampicin monoresistant/multidrug-resistant TB (Rr/MDR-TB) of 21.6% (95% CI 14.7-28.4), 10.8% (95% CI 5.6-16.0%), and 2.9% (95% CI 0.1-5.7%), respectively. The DR-TB rates did not differ significantly between 2006-2013, 2014-2018, and 2019-2021 (p > 0.05). Two pre-extensively DR-TB (pre-XDR) cases with fluoroquinolone resistance were detected after 2014.
Conclusion: The prevalence of DR-TB in Thai children was stable. However, one-tenth of DR-TB cases confirmed with DST were Hr-TB, which required adjustment of the treatment regimen. The pre-XDR cases should be closely monitored.
Keywords: DR-TB, Drug resistant tuberculosis; Hr-TB, Isoniazid-monoresistant tuberculosis; MDR-TB, Multidrug-resistant tuberculosis; Pre-XDR-TB, Pre-extensively drug-resistant tuberculosis; Rr-TB, Rifampicin-monoresistant tuberculosis; children; drug-resistant tuberculosis; fluoroquinolone; isoniazid-monoresistant tuberculosis (Hr-TB); multidrug-resistant tuberculosis (MDR-TB); pre-extensively drug-resistant tuberculosis (pre-XDR-TB); rifampicin-monoresistant tuberculosis (Rr-TB).
© 2022 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Chumpa N, Kawkitinarong K, Rotcheewaphan S, Sawatpanich A, Petsong S, Tumwasorn S, et al. Evaluation of Anyplex™ II MTB/MDR kit's performance to rapidly detect isoniazid and rifampicin resistant Mycobacterium tuberculosis from various clinical specimens. Mol Biol Rep. 2020;47(4):2501–2508. - PubMed
-
- Dheda K, Gumbo T, Maartens G, Dooley KE, Murray M, Furin J, et al. The Lancet Respiratory Medicine Commission: 2019 update: epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant and incurable tuberculosis. Lancet Respir Med. 2019;7(9):820–826. - PubMed
-
- Division of Tuberculosis, Department of Disease Control, Ministry of Public Health . Agricultural Cooperative Printing Demonstrations of Thai, Ltd.; Bangkok: 2015. Guideline for programmatic management of drug-resistant tuberculosis.
LinkOut - more resources
Full Text Sources
Miscellaneous