Protocol for a cluster randomised controlled feasibility study of Prehospital Optimal Shock Energy for Defibrillation (POSED)
- PMID: 36238581
- PMCID: PMC9550652
- DOI: 10.1016/j.resplu.2022.100310
Protocol for a cluster randomised controlled feasibility study of Prehospital Optimal Shock Energy for Defibrillation (POSED)
Abstract
Aims: The Prehospital Optimal Shock Energy for Defibrillation (POSED) study will assess the feasibility of conducting a cluster randomised controlled study of clinical effectiveness in UK ambulance services to identify the optimal shock energy for defibrillation.
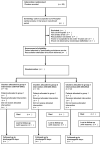
Methods: POSED is a pragmatic, allocation concealed, open label, cluster randomised, controlled feasibility study. Defibrillators within a single UK ambulance service will be randomised in an equal ratio to deliver one of three shock strategies 120-150-200 J, 150-200-200 J, 200-200-200 J. Consecutive adults (≥18 years) presenting with out of hospital cardiac arrest requiring defibrillation will be eligible. The study plans to enrol 90 patients (30 in each group). Patients (or their relatives for non-survivors) will be informed about trial participation after the initial emergency has resolved. Survivors will be invited to consent to participate in follow-up (i.e., at 30 days or discharge).The primary feasibility outcome is the proportion of eligible patients who receive the randomised study intervention. Secondary feasibility outcomes will include recruitment rate, adherence to allocated treatment and data completeness. Clinical outcomes will include Return of an Organised Rhythm (ROOR) at 2 minutes post-shock, refibrillation rate, Return of Spontaneous Circulation (ROSC) at hospital handover, survival and neurological outcome at 30 days.
Conclusion: The POSED study will assess the feasibility of a large-scale trial and explore opportunities to optimise the trial protocol.Trial registration: ISRCTN16327029.
Keywords: AE, Adverse Event; AOR, Adjusted Odds Ratio; B-CPR, Bystander CPR; BTE, Biphasic Truncated Exponential waveform; CAD, Computer Aided Despatch; CONSORT, CONsolidated Standards Of Reporting Trials; CPMS, Central Portfolio Management System; CPR, Cardiopulmonary Resuscitation; CRF, Case Report Form; Cardiopulmonary Resuscitation; Defibrillation; Electric Countershock; Feasibility study; GCP, Good Clinical Practice; HRA, Health Research Authority; ICA, Integrated Clinical and practitioner Academic programme; ILCOR, International Liaison Committee on Resuscitation; ISRCTN, International Standard Registered Clinical/social sTudy Number; J, Joules; JRCALC, Joint Royal Colleges Ambulance Liaison Committee; NIHR, National Institute for Health and care Research; OHCA, Out-of-Hospital Cardiac Arrest; OR, Odds Ratio; Out-of-Hospital Cardiac Arrest; PEA, Pulseless Electrical Activity; POSED, Prehospital Optimal Shock Energy for Defibrillation; PPI, Patient and Public Involvement; REC, Research Ethics Committee; RFA, Rankin Focused Assessment; ROOR, Return of Organised Rhythm; ROSC, Return of Spontaneous Circulation; SMG, Study Management Group; SOC, Study Oversight Committee; SPIRIT, Standard Protocol Items: Recommendations for Intervention Trials; ToF, Termination of Fibrillation; VF, Ventricular Fibrillation; Ventricular Fibrillation; WCTU, Warwick Clinical Trials Unit; ePR, Electronic Patient Record; mRS, Modified Rankin Scale; pVT, Pulseless Ventricular Tachycardia.
© 2022 The Author(s).
Conflict of interest statement
HP reports research funding from National Institute for Health and Care Research. CDD, RL, FM, AC, MAS, PK, AD, DES report no conflicts of interest. GDP reports research funding from National Institute for Health and Care Research, British Heart Foundation and Resuscitation Council UK. He has volunteer roles with Resuscitation Council UK, European Resuscitation Council and the International Liaison Committee on Resuscitation. He is an editor for the journals Resuscitation and Resuscitation Plus.
References
-
- Kiguchi T., Okubo M., Nishiyama C., et al. Out-of-hospital cardiac arrest across the World: First report from the International Liaison Committee on Resuscitation (ILCOR) Resuscitation. 2020;152:39–49. - PubMed
-
- Valenzuela T.D., Roe D.J., Cretin S., Spaite D.W., Larsen M.P. Estimating Effectiveness of Cardiac Arrest Interventions: A Logistic Regression Survival Model. Circulation. 1997;96:3308–3313. - PubMed
-
- Holmen J., Hollenberg J., Claesson A., et al. Survival in ventricular fibrillation with emphasis on the number of defibrillations in relation to other factors at resuscitation. Resuscitation. 2017;133:33–38. - PubMed
-
- Matsuyama T., Kitamura T., Kiyohara K., et al. Impact of cardiopulmonary resuscitation duration on neurologically favourable outcome after out-of-hopstial cardiac arrest: A population-based study in Japan. Resuscitation. 2017;113:1–7. - PubMed
-
- Soar J, Deakin, C.D., Nolan, J.P., et al. Resuscitation Council (UK) Adult advanced life support guidelines 2021. Available from: https://www.resus.org.uk/library/2021-resuscitation-guidelines/adult-adv....
LinkOut - more resources
Full Text Sources
Miscellaneous