Drug-Induced Photosensitivity: Clinical Types of Phototoxicity and Photoallergy and Pathogenetic Mechanisms
- PMID: 36238932
- PMCID: PMC9552952
- DOI: 10.3389/falgy.2022.876695
Drug-Induced Photosensitivity: Clinical Types of Phototoxicity and Photoallergy and Pathogenetic Mechanisms
Abstract
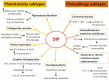
Drug-induced photosensitivity (DIP) is a common cutaneous adverse drug reaction, resulting from the interaction of ultraviolet radiations, mostly ultraviolet A, with drugs. DIP includes phototoxicity and photoallergy. A phototoxic reaction is obtained when topical and systemic drugs or their metabolites absorb light inducing a direct cellular damage, while a photoallergic reaction takes place when the interaction between drugs and ultraviolet radiations causes an immune cutaneous response. Clinically, phototoxicity is immediate and appears as an exaggerated sunburn, whereas photoallergy is a delayed eczematous reaction. DIP may show several clinical subtypes. In this mini-review we report the pathogenetic mechanisms and causative drugs of DIP. We offer a detailed description of DIP clinical features in its classical and unusual subtypes, such as hyperpigmentation/dyschromia, pseudoporphyria, photo-onycolysis, eruptive teleangiectasia, pellagra-like reaction, lichenoid reaction, photodistributed erythema multiforme and subacute/chronic cutaneous lupus erythematosus. We described how physicians may early recognize and manage DIP, including diagnostic tests to rule out similar conditions. We made suggestions on how to improve sun exposure behaviors of patients at risk of DIP by means of an aware use of sunscreens, protective clothing and recent technologic tools. We highlighted the lack of sun safety programs addressed to patients at risk of DIP, who need a formal education about their condition.
Keywords: drug reaction; pathogenetic mechanisms; patient education; photoallergy; photosensitivity; phototoxicity.
Copyright © 2022 Di Bartolomeo, Irrera, Campo, Borgia, Motolese, Vaccaro, Squadrito, Altavilla, Condorelli, Motolese and Vaccaro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources