Zavegepant nasal spray for the acute treatment of migraine: A Phase 2/3 double-blind, randomized, placebo-controlled, dose-ranging trial
- PMID: 36239038
- PMCID: PMC9827820
- DOI: 10.1111/head.14389
Zavegepant nasal spray for the acute treatment of migraine: A Phase 2/3 double-blind, randomized, placebo-controlled, dose-ranging trial
Abstract
Objective: Evaluate the efficacy, safety, and tolerability of zavegepant nasal spray in the acute treatment of migraine.
Background: Calcitonin gene-related peptide-targeting agents are a novel class of therapeutics for migraine, but none are currently available as a nonoral option for acute treatment. Zavegepant, a high-affinity, selective, and structurally unique calcitonin gene-related peptide-receptor antagonist in late-stage development, is formulated as a nasal spray for the acute treatment of migraine.
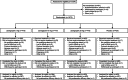
Methods: This randomized, dose-ranging, placebo-controlled, Phase 2/3 trial in adults aged ≥18 years with migraine (NCT03872453) was conducted at US study sites. Participants were randomized by an interactive web response system and treated a single attack of moderate to severe pain intensity with zavegepant nasal spray 5, 10, 20 mg, or placebo. Coprimary efficacy endpoints were pain freedom and freedom from the most bothersome symptom at 2 h postdose.
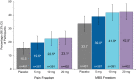
Results: Of the 1673 participants aged 18 to 79 years who were randomized, 1588 were treated with study medication, and 1581 (mean age 40.8 years, 85.5% female) were analyzed for efficacy: zavegepant 5 mg (n = 387), 10 mg (n = 391), 20 mg (n = 402), and placebo (n = 401). Zavegepant 10 and 20 mg were more effective than placebo on the coprimary endpoints of pain freedom at 2 h postdose (placebo: 15.5% [98.3% confidence interval (CI), 11.1, 19.8]; 10 mg: 22.5% [98.3% CI, 17.5, 27.6; p = 0.0113]; 20 mg: 23.1% [98.3% CI, 18.1, 28.2; p = 0.0055]) and freedom from the most bothersome symptom at 2 h postdose (placebo: 33.7% [98.3% CI, 28.0, 39.3]; 10 mg: 41.9% [98.3% CI, 36.0, 47.9; p = 0.0155]; 20 mg: 42.5% [98.3% CI, 36.6, 48.4; p = 0.0094]). Findings for the 5 mg dose were not significant. The most common treatment-emergent adverse events with zavegepant 10 and 20 mg and placebo were dysgeusia (13.5% to 16.1% vs. 3.5%), nausea (2.7% to 4.1% vs. 0.5%), and nasal discomfort (1.3% to 5.2% vs. 0.2%). Most adverse events were mild or moderate and resolved without treatment. There was no signal of hepatotoxicity.
Conclusion: Zavegepant nasal spray, in single doses of 10 or 20 mg, was effective for the acute treatment of migraine, with a favorable safety profile. Additional research is needed to confirm its potential as a nonoral medication for the acute treatment of migraine.
Keywords: CGRP; acute treatment; intranasal; migraine; nasal spray; zavegepant.
© 2022 Biohaven Pharmaceuticals, Inc. Headache: The Journal of Head and Face Pain published by Wiley Periodicals LLC on behalf of American Headache Society.
Conflict of interest statement
Robert Croop, MD, is employed by and holds stock/stock options in Biohaven Pharmaceuticals. Jennifer Madonia, MS, PA‐C, is employed by and holds stock/stock options in Biohaven Pharmaceuticals. Alexandra Thiry, PhD, is employed by and holds stock/stock options in Biohaven Pharmaceuticals. Micaela Forshaw, MPH, is employed by and holds stock/stock options in Biohaven Pharmaceuticals. David A. Stock, PhD, is employed by and holds stock/stock options in Biohaven Pharmaceuticals. Abigail Murphy, BA, is employed by and holds stock/stock options in Biohaven Pharmaceuticals. Vladimir Coric, MD, is employed by and holds stock/stock options in Biohaven Pharmaceuticals. Richard B. Lipton, MD, serves on the editorial board of
Figures
Comment in
-
A non-oral gepant for acute treatment of migraine.Headache. 2022 Oct;62(9):1075-1076. doi: 10.1111/head.14397. Epub 2022 Sep 16. Headache. 2022. PMID: 36112070 No abstract available.
-
Neues Nasenspray zur Akuttherapie bei Migräne.MMW Fortschr Med. 2023 Jan;165(1):27. doi: 10.1007/s15006-023-2259-7. MMW Fortschr Med. 2023. PMID: 36648657 Review. German. No abstract available.
References
-
- Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3‐20. - PubMed
-
- Lipton RB, Munjal S, Alam A, et al. Migraine in America Symptoms and Treatment (MAST) study: baseline study methods, treatment patterns, and gender differences. Headache. 2018;58:1408‐1426. - PubMed
-
- Silberstein SD. Practice parameter: evidence‐based guidelines for migraine headache (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754‐762. - PubMed
-
- Ailani J, Burch RC, Robbins MS, Society tBoDotAH . The American Headache Society Consensus Statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61:1021‐1039. - PubMed
-
- Lipton RB, Hamelsky SW, Dayno JM. What do patients with migraine want from acute migraine treatment? Headache. 2002;42(Suppl 1):3‐9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

