Anti-CD38 monoclonal antibody interference with blood compatibility testing: Differentiating isatuximab and daratumumab via functional epitope mapping
- PMID: 36239134
- PMCID: PMC9828815
- DOI: 10.1111/trf.17137
Anti-CD38 monoclonal antibody interference with blood compatibility testing: Differentiating isatuximab and daratumumab via functional epitope mapping
Abstract
Background: There are two FDA-approved anti-CD38 monoclonal antibodies for treatment of multiple myeloma: isatuximab and daratumumab. Owing to expression of CD38 on reagent red blood cells (RBCs), these antibodies interfere with indirect antiglobulin tests (IATs). We sought to understand differences in such interference by performing binding experiments.
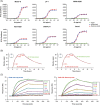
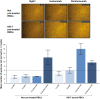
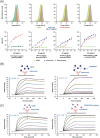
Study design and methods: In vitro experiments to compare the binding to RBCs of isatuximab and daratumumab alone or in the presence of a mouse anti-human CD38 antibody (HB-7 or AT13/5) or a nicotinamide adenine dinucleotide-analog CD38 inhibitor were performed and quantified by flow cytometry, imaging, mass spectrometry, surface plasmon resonance, and LigandTracer technologies. Serologic testing was performed on plasma samples spiked with isatuximab or daratumumab.
Results: CD38 expressed on RBCs can be directly bound by daratumumab, whereas isatuximab requires a co-factor, such as HB-7, AT13/5, or a CD38 inhibitor, suggesting that the isatuximab epitope on RBCs is masked in vitro. Daratumumab samples more frequently showed interference and had stronger reactions than isatuximab samples. Dithiothreitol treatment was equally effective in mitigating the interference caused by either drug.
Discussion: Both isatuximab and daratumumab interfere with IATs but at different magnitudes, reflecting distinct binding to CD38 on RBCs. From the binding studies, we conclude that the isatuximab epitope on RBCs is masked in vitro and binding requires a certain CD38 conformation or co-factor. This circumstance may explain why interference is seen only in a subset of patients receiving isatuximab when compared with interference seen in most patients on daratumumab therapy.
© 2022 The Authors. Transfusion published by Wiley Periodicals LLC on behalf of AABB.
Conflict of interest statement
Btissam Chami: No relevant financial relationships to disclose. Makoto Okuda: No relevant financial relationships to disclose. Morvarid Moayeri: Institution support—Sanofi. France Pirenne: No relevant financial relationships to disclose. Yoko Hidaka: No relevant financial relationships to disclose. Ashok Nambiar: Institution support—Sanofi. Zhili Song: Employed by Sanofi; may hold stock and/or stock options in the company. Olivier Bedel: Employed by Sanofi at the time of study. Bailin Zhang: Employed by Sanofi; may hold stock and/or stock options in the company. Joern Hopke: Employed by Sanofi; may hold stock and/or stock options in the company. Gejing Deng: Employed by Sanofi; may hold stock and/or stock options in the company. Chen Zhu: Employed by Sanofi; may hold stock and/or stock options in the company. Sandrine Macé: Employed by Sanofi; may hold stock and/or stock options in the company. Marielle Chiron: Employed by Sanofi; may hold stock and/or stock options in the company. Francisco Adrian: Employed by Sanofi at the time of study. Taro Fukao: Employed by Sanofi; may hold stock and/or stock options in the company. Frank Basile: Employed by Sanofi at the time of study. Thomas Martin: Research funding—Sanofi, Seattle Genetics, Amgen, Janssen, GSK.
Figures


References
-
- Jiang H, Acharya C, An G, Zhong M, Feng X, Wang L, et al. SAR650984 directly induces multiple myeloma cell death via lysosomal‐associated and apoptotic pathways, which is further enhanced by pomalidomide. Leukemia. 2016;30(2):399–408. - PubMed
-
- Sarclisa (isatuximab‐irfc) injection for intravenous use . Prescribing information. sanofi‐aventis U.S. LLC. Available from: https://products.sanofi.us/Sarclisa/sarclisa.pdf. Accessed July 21, 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

