Neutrophil activation and neutrophil extracellular traps (NETs) in COVID-19 ARDS and immunothrombosis
- PMID: 36239164
- PMCID: PMC9874644
- DOI: 10.1002/eji.202250010
Neutrophil activation and neutrophil extracellular traps (NETs) in COVID-19 ARDS and immunothrombosis
Abstract
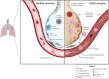
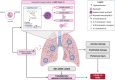
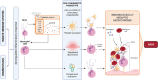
Acute respiratory distress syndrome (ARDS) is an acute inflammatory condition with a dramatic increase in incidence since the beginning of the coronavirus disease 19 (COVID-19) pandemic. Neutrophils play a vital role in the immunopathology of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection by triggering the formation of neutrophil extracellular traps (NETs), producing cytokines including interleukin-8 (CXCL8), and mediating the recruitment of other immune cells to regulate processes such as acute and chronic inflammation, which can lead to ARDS. CXCL8 is involved in the recruitment, activation, and degranulation of neutrophils, and therefore contributes to inflammation amplification and severity of disease. Furthermore, activation of neutrophils also supports a prothrombotic phenotype, which may explain the development of immunothrombosis observed in COVID-19 ARDS. This review aims to describe hyperinflammatory ARDS due to SARS-CoV-2 infection. In addition, we address the critical role of polymorphonuclear neutrophils, inflammatory cytokines, and the potential targeting of CXCL8 in treating the hyperinflammatory ARDS population.
Keywords: ARDS; COVID-19; CXCL8; immunomodulators.
© 2022 The Authors. European Journal of Immunology published by Wiley-VCH GmbH.
Conflict of interest statement
R.B. and A.K. are consultants for Dompé U.S. Inc. E.M.G., M.C.C., M.Z., C.M., F.M., and M.A. are full‐time employees of Dompé farmaceutici S.p.A.
Figures
References
-
- Pham, T. and Rubenfeld, G.D. , Fifty years of research in ARDS. The epidemiology of acute respiratory distress syndrome. A 50th birthday review. Am J Respir Crit Care Med. 2017. 195(7): 860‐70. - PubMed
-
- Johnston, C.J. , Rubenfeld, G.D. and Hudson, L.D. , Effect of age on the development of ARDS in trauma patients. Chest. 2003. 124(2): 653‐9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

