Multiorgan neutrophilic inflammation in a Border Collie with "trapped" neutrophil syndrome
- PMID: 36239343
- PMCID: PMC9708390
- DOI: 10.1111/jvim.16567
Multiorgan neutrophilic inflammation in a Border Collie with "trapped" neutrophil syndrome
Abstract
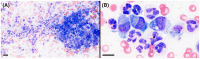
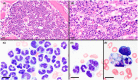
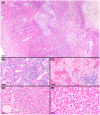
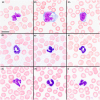
Trapped neutrophil syndrome is a rare congenital disease recognized in Border Collies and is characterized by persistent neutropenia with myeloid hyperplasia. The mechanism of neutropenia has not been described. We document the case of a young Border Collie diagnosed with trapped neutrophil syndrome based on clinical features, blood and bone marrow evaluation, and presence of the associated homozygous mutation. Results from flow cytometric and storage studies suggested lower neutrophil survival time. The dog had substantial neutrophilic inflammation in multiple organs, indicating that neutrophils could leave the marrow and enter tissues, making the term "trapped" neutrophil syndrome a misnomer.
Keywords: Cohen syndrome; VPS13B; bone marrow; ineffective myelopoiesis; neutropenia.
© 2022 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of Wiley Periodicals LLC. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Conflict of interest statement
Authors declare no conflict of interest.
Figures


References
-
- Allan FJ, Thompson KG, Jones BR, Burbidge HM, McKinley R. Neutropenia with a probable hereditary basis in border collies. N Z Vet J. 1996;44(2):67‐72. - PubMed
-
- Hegler AK, Grooters AM, Dehghanpir SD, Gallaher RA, Gaschen LE. Trapped neutrophil syndrome in a border collie. J Am Anim Hosp Assoc. 2020;56(3):e563‐04. - PubMed
-
- Gans Z. Confirmed trapped neutrophil syndrome in a border collie puppy in Israel. Isr J Vet Med. 2015;70(2):45‐48.
-
- Mason SL, Jepson R, Maltman M, Batchelor DJ. Presentation and management of trapped neutrophil syndrome (TNS) in UK border collies. J Small Anim Pract. 2014;55(1):57‐60. - PubMed
-
- Mizukami K, Shoubudani T, Nishimoto S, et al. Trapped neutrophil syndrome in a border collie dog: clinical, clinico‐pathologic, and molecular findings. J Vet Med Sci. 2012;74(6):797‐800. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
