Loss of function of the maternal membrane oestrogen receptor ERα alters expansion of trophoblast cells and impacts mouse fertility
- PMID: 36239412
- PMCID: PMC9720743
- DOI: 10.1242/dev.200683
Loss of function of the maternal membrane oestrogen receptor ERα alters expansion of trophoblast cells and impacts mouse fertility
Abstract
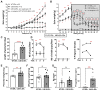
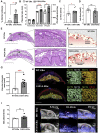
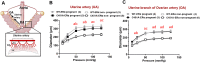
The binding of 17β-oestradiol to oestrogen receptor alpha (ERα) plays a crucial role in the control of reproduction, acting through both nuclear and membrane-initiated signalling. To study the physiological role of membrane ERα in the reproductive system, we used the C451A-ERα mouse model with selective loss of function of membrane ERα. Despite C451A-ERα mice being described as sterile, daily weighing and ultrasound imaging revealed that homozygous females do become pregnant, allowing the investigation of the role of ERα during pregnancy for the first time. All neonatal deaths of the mutant offspring mice resulted from delayed parturition associated with failure in pre-term progesterone withdrawal. Moreover, pregnant C451A-ERα females exhibited partial intrauterine embryo arrest at about E9.5. The observed embryonic lethality resulted from altered expansion of Tpbpa-positive spiral artery-associated trophoblast giant cells into the utero-placental unit, which is associated with an imbalance in expression of angiogenic factors. Together, these processes control the trophoblast-mediated spiral arterial remodelling. Hence, loss of membrane ERα within maternal tissues clearly alters the activity of invasive trophoblast cells during placentogenesis. This previously unreported function of membrane ERα could open new avenues towards a better understanding of human pregnancy-associated pathologies.
Keywords: Fertility; Membrane signalling; Oestrogen receptor ERα (ESR1); Parturition; Spiral arterial remodelling; Trophoblast cells.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

References
-
- Adlanmerini, M., Solinhac, R., Abot, A., Fabre, A., Raymond-Letron, I., Guihot, A.-L., Boudou, F., Sautier, L., Vessières, E., Kim, S. H.et al. (2014). Mutation of the palmitoylation site of estrogen receptor α in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc. Natl. Acad. Sci. USA 111, E283-E290. 10.1073/pnas.1322057111 - DOI - PMC - PubMed
-
- Adlanmerini, M., Fébrissy, C., Zahreddine, R., Vessières, E., Buscato, M., Solinhac, R., Favre, J., Anquetil, T., Guihot, A.-L., Boudou, F.et al. (2020). Mutation of Arginine 264 on ERα (Estrogen Receptor Alpha) selectively abrogates the rapid signaling of estradiol in the endothelium without altering fertility. Arterioscler. Thromb. Vasc. Biol. 40, 2143-2158. 10.1161/ATVBAHA.120.314159 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

