An In Vivo Mouse Model for Chronic Inflammation-Induced Immune Suppression: A "Factory" for Myeloid-Derived Suppressor Cells (MDSCs)
- PMID: 36239438
- PMCID: PMC11648823
- DOI: 10.1002/cpz1.558
An In Vivo Mouse Model for Chronic Inflammation-Induced Immune Suppression: A "Factory" for Myeloid-Derived Suppressor Cells (MDSCs)
Abstract
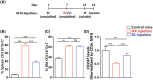
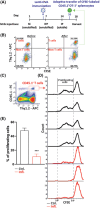
Myeloid-derived suppressor cells (MDSCs) represent a heterogeneous population of immature myeloid cells known to play a role in perpetuating a wide range of pathologies, such as chronic infections, autoimmune diseases, and cancer. MDSCs were first identified in mice by the markers CD11b+ Gr1+ , and later, based on their morphology, they were classified into two subsets: polymorphonuclear MDSCs, identified by the markers CD11b+ Ly6G+ Ly6CLow , and monocytic MDSCs, detected as being CD11b+ Ly6G- Ly6CHi . MDSCs are studied as immunosuppressive cells in various diseases characterized by chronic inflammation and are associated with disease causes/triggers such as pathogens, autoantigens, and cancer. Therefore, different diseases may diversely affect MDSC metabolism, migration, and differentiation, thus influencing the generated MDSC functional features and ensuing suppressive environment. In order to study MDSCs in a pathology-free environment, we established and calibrated a highly reproducible mouse model that results in the development of chronic inflammation, which is the major cause of MDSC accumulation and immune suppression. The model presented can be used to study MDSC phenotypes, functional diversity, and plasticity. It also permits study of MDSC migration from the bone marrow to peripheral lymphatic and non-lymphatic organs and MDSC crosstalk with extrinsic factors, both in vivo and ex vivo. Furthermore, this model can serve as a platform to assess the effects of anti-MDSC modalities. © 2022 The Authors. Current Protocols published by Wiley Periodicals LLC. Basic Protocol: Repetitive M.tb immunizations for the induction of chronic inflammation Alternate Protocol 1: Creating a lower grade of inflammation by changing the site of immunization Alternate Protocol 2: In vivo evaluation of immune status Support Protocol 1: Preparation of reconstituted M.tb aliquots and M.tb-IFA emulsions for each of the three injections Support Protocol 2: Preparation of an ovalbumin lentiviral expression vector Support Protocol 3: Fluorescence titering assay for the lentiviral expression vector Support Protocol 4: Spleen excision, tissue dissociation, and preparation of a single-cell suspension Support Protocol 5: Labeling of splenocytes with CFSE proliferation dye.
Keywords: M-MDSCs; PMN-MDSCs; chronic inflammation; immune suppression; myeloid cells.
© 2022 The Authors. Current Protocols published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bronstein‐Sitton, N. , Cohen‐Daniel, L. , Vaknin, I. , Ezernitchi, A. V. , Leshem, B. , Halabi, A. , … Baniyash, M. (2003). Sustained exposure to bacterial antigen induces interferon‐γ‐dependent T cell receptor ζ down‐regulation and impaired T cell function. Nature Immunology, 4, 957–964. doi: 10.1038/ni975 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

