Shipping and Logistics Considerations for Regenerative Medicine Therapies
- PMID: 36239619
- PMCID: PMC9562819
- DOI: 10.1093/stcltm/szab025
Shipping and Logistics Considerations for Regenerative Medicine Therapies
Abstract
Advances in regenerative medicine manufacturing continue to be a priority for achieving the full commercial potential of important breakthrough therapies. Equally important will be the establishment of distribution chains that support the transport of live cells and engineered tissues and organs resulting from these advanced biomanufacturing processes. The importance of a well-managed distribution chain for products requiring specialized handling procedures was highlighted during the COVID-19 pandemic and serves as a reminder of the critical role of logistics and distribution in the success of breakthrough therapies. This perspective article will provide insight into current practices and future considerations for creating global distribution chains that facilitate the successful deployment of regenerative medicine therapies to the vast number of patients that would benefit from them worldwide.
Keywords: distribution; manufacturing; regenerative medicine; shipping and logistics.
© The Author(s) 2022. Published by Oxford University Press.
Figures

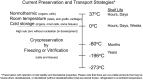
References
-
- FDA authorizes Pfizer-BioNTech COVID-19 vaccine. Med Lett Drugs Ther. 2021;63(1615):1–2. - PubMed
-
- FDA authorizes Moderna COVID-19 vaccine. Med Lett Drugs Ther. 2021;63(1616):9–10. - PubMed
-
- U.S. Food and Drug Administration. J&J COVID-19 vaccine: emergency use authorization. Published Feb 27, 2021. Accessed July 18, 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise...
-
- Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine: storage and handling. Published 2021. Accessed July 18, 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/downloads/s...
-
- Centers for Disease Control and Prevention. Moderna COVID-19 vaccine: storage and handling. Published 2021. Accessed July 18, 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/downloads/...
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

