Cardiovascular magnetic resonance in light-chain amyloidosis to guide treatment
- PMID: 36239754
- PMCID: PMC9712028
- DOI: 10.1093/eurheartj/ehac363
Cardiovascular magnetic resonance in light-chain amyloidosis to guide treatment
Abstract
Aims: To assess the ability of cardiovascular magnetic resonance (CMR) to (i) measure changes in response to chemotherapy; (ii) assess the correlation between haematological response and changes in extracellular volume (ECV); and (iii) assess the association between changes in ECV and prognosis over and above existing predictors.
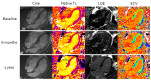
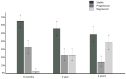
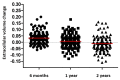
Methods and results: In total, 176 patients with cardiac AL amyloidosis were assessed using serial N-terminal pro-B-type natriuretic peptide (NT-proBNP), echocardiography, free light chains and CMR with T1 and ECV mapping at diagnosis and subsequently 6, 12, and 24 months after starting chemotherapy. Haematological response was graded as complete response (CR), very good partial response (VGPR), partial response (PR), or no response (NR). CMR response was graded by changes in ECV as progression (≥0.05 increase), stable (<0.05 change), or regression (≥0.05 decrease). At 6 months, CMR regression was observed in 3% (all CR/VGPR) and CMR progression in 32% (61% in PR/NR; 39% CR/VGPR). After 1 year, 22% had regression (all CR/VGPR), and 22% had progression (63% in PR/NR; 37% CR/VGPR). At 2 years, 38% had regression (all CR/VGPR), and 14% had progression (80% in PR/NR; 20% CR/VGPR). Thirty-six (25%) patients died during follow-up (40 ± 15 months); CMR response at 6 months predicted death (progression hazard ratio 3.82; 95% confidence interval 1.95-7.49; P < 0.001) and remained prognostic after adjusting for haematological response, NT-proBNP and longitudinal strain (P < 0.01).
Conclusions: Cardiac amyloid deposits frequently regress following chemotherapy, but only in patients who achieve CR or VGPR. Changes in ECV predict outcome after adjusting for known predictors.
Keywords: Amyloidosis; CMR; ECV; T1 mapping.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Cardiology.
Conflict of interest statement
Conflict of interest: M.F. is supported by a British Heart Foundation Intermediate Clinical Research Fellowship (FS/18/21/33447). The other authors declare that there is no conflict of interest.
Figures



Comment in
-
The challenge of managing patients with light-chain cardiac amyloidosis: the value of cardiac magnetic resonance as a guide to the treatment response.Eur Heart J. 2022 Dec 1;43(45):4736-4738. doi: 10.1093/eurheartj/ehac526. Eur Heart J. 2022. PMID: 36269640 No abstract available.
References
-
- Cohen O, Ismail A, Manwani R, Ravichandran S, Foard D, Mahmood S, et al. . Global longitudinal strain predicts survival and response in patients with systemic AL amyloidosis. Analysis of 915 patients from the ALchemy prospective trial [abstract]. Eur Heart J 2020;41:856. doi:10.1093/eurheartj/ehaa452 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

