Association Between Time Interval from COVID-19 Vaccination to In Vitro Fertilization and Pregnancy Rate After Fresh Embryo Transfer
- PMID: 36239937
- PMCID: PMC9568801
- DOI: 10.1001/jamanetworkopen.2022.36609
Association Between Time Interval from COVID-19 Vaccination to In Vitro Fertilization and Pregnancy Rate After Fresh Embryo Transfer
Abstract
Importance: There is a lack of information regarding the need to postpone conception after COVID-19 vaccination.
Objective: To investigate the time interval between the first dose of inactivated COVID-19 vaccine and in vitro fertilization (IVF) treatment as well as the rate of pregnancy after a fresh embryo transfer.
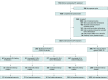
Design, setting, and participants: This cohort study was conducted at a single public IVF center in China. Female patients aged 20 to 47 years and undergoing IVF treatment were consecutively registered from May 1 to December 22, 2021, with follow-up until March 31, 2022. Patients with SARS-CoV-2 infection before or during IVF treatment and those who underwent 2 or more IVF treatments, received the noninactivated or unknown COVID-19 vaccine, or did not have a fresh embryo transfer were excluded from this study.
Exposures: The vaccinated group (subdivided into 4 subgroups of time interval from first vaccination to fertilization treatment: ≤30 days, 31-60 days, 61-90 days, and ≥91 days) and nonvaccinated group.
Main outcomes and measures: Risk ratios (RRs) for the association between the time interval and ongoing pregnancy (pregnancy continued at least 12 weeks).
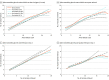
Results: A total of 3052 female patients (mean [SD] age, 31.45 [3.96] years) undergoing IVF treatment were analyzed in this study. There were 667 vaccinated patients receiving IVF (35 were vaccinated ≤30 days, 58 were vaccinated 31-60 days, 105 were vaccinated 61-90 days, and 469 were vaccinated ≥91 days before fertilization treatment), and 2385 unvaccinated patients receiving treatment. The ovarian stimulation and laboratory parameters were similar among all groups. Ongoing pregnancy was significantly lower in the 30 days or less subgroup (34.3% [12 of 35]; adjusted RR [aRR], 0.61; 95% CI, 0.33-0.91) and the 31 to 60 days' subgroup (36.2% [21 of 58]; aRR, 0.63; 95% CI, 0.42-0.85). A slightly but not statistically lower rate was found in the 61 to 90 days' subgroup, and no reduced risk for ongoing pregnancy in the 91 days or more subgroup was observed (56.3% [264 of 469]; aRR, 0.96; 95% CI, 0.88-1.04) compared with the unvaccinated group (60.3% [1439 of 2385], as reference).
Conclusions and relevance: Findings of this study suggest that receipt of the first inactivated COVID-19 vaccine dose 60 days or less before fertilization treatment is associated with a reduced rate of pregnancy. In patients undergoing IVF treatment with a fresh embryo transfer, the procedure may need to be delayed until at least 61 days after COVID-19 vaccination.
Conflict of interest statement
Figures


Comment in
-
In Vitro Fertilization Outcomes After Inactivated COVID-19 Vaccine.JAMA Netw Open. 2022 Oct 3;5(10):e2236618. doi: 10.1001/jamanetworkopen.2022.36618. JAMA Netw Open. 2022. PMID: 36239944 No abstract available.
References
-
- ASRM COVID-19 Task Force . ASRM patient management and clinical recommendations during the Coronavirus (COVID-19) pandemic. ASRM Bulletin. April 20, 2022. Accessed September 9, 2022. https://www.asrm.org/news-and-publications/news-and-research/press-relea...
-
- Centers for Disease Control and Prevention . Planning for pregnancy: COVID-19 vaccines for people who would like to have a baby. Accessed September 9, 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/planning-for-pregnanc...
-
- European Society of Human Reproduction and Embryology COVID-19 Working Group . COVID-19 vaccination and assisted reproduction. January 12, 2021. Accessed September 9, 2022. https://www.eshre.eu/-/media/sitecore-files/Position-statements/COVID19-...
-
- Qiao J; Expert Group for Beijing Human Assisted Reproductive Technology Center for Quality Control and Improvement . COVID-19 vaccination strategy for planning pregnancy and assisted reproductive technology treatment: expert recommendations [in Chinese]. Chin J Reprod Contracep. 2021;41(4):296-299.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

