Colorectal Cancer Metastases in the Liver Establish Immunosuppressive Spatial Networking between Tumor-Associated SPP1+ Macrophages and Fibroblasts
- PMID: 36239989
- PMCID: PMC9811165
- DOI: 10.1158/1078-0432.CCR-22-2041
Colorectal Cancer Metastases in the Liver Establish Immunosuppressive Spatial Networking between Tumor-Associated SPP1+ Macrophages and Fibroblasts
Abstract
Purpose: The liver is the most frequent metastatic site for colorectal cancer. Its microenvironment is modified to provide a niche that is conducive for colorectal cancer cell growth. This study focused on characterizing the cellular changes in the metastatic colorectal cancer (mCRC) liver tumor microenvironment (TME).
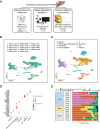
Experimental design: We analyzed a series of microsatellite stable (MSS) mCRCs to the liver, paired normal liver tissue, and peripheral blood mononuclear cells using single-cell RNA sequencing (scRNA-seq). We validated our findings using multiplexed spatial imaging and bulk gene expression with cell deconvolution.
Results: We identified TME-specific SPP1-expressing macrophages with altered metabolism features, foam cell characteristics, and increased activity in extracellular matrix (ECM) organization. SPP1+ macrophages and fibroblasts expressed complementary ligand-receptor pairs with the potential to mutually influence their gene-expression programs. TME lacked dysfunctional CD8 T cells and contained regulatory T cells, indicative of immunosuppression. Spatial imaging validated these cell states in the TME. Moreover, TME macrophages and fibroblasts had close spatial proximity, which is a requirement for intercellular communication and networking. In an independent cohort of mCRCs in the liver, we confirmed the presence of SPP1+ macrophages and fibroblasts using gene-expression data. An increased proportion of TME fibroblasts was associated with the worst prognosis in these patients.
Conclusions: We demonstrated that mCRC in the liver is characterized by transcriptional alterations of macrophages in the TME. Intercellular networking between macrophages and fibroblasts supports colorectal cancer growth in the immunosuppressed metastatic niche in the liver. These features can be used to target immune-checkpoint-resistant MSS tumors.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures





References
-
- Andres A, Mentha G, Adam R, Gerstel E, Skipenko OG, Barroso E, et al. . Surgical management of patients with colorectal cancer and simultaneous liver and lung metastases. Br J Surg 2015;102:691–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

