Mechanisms of TNF-independent RIPK3-mediated cell death
- PMID: 36240069
- PMCID: PMC9704539
- DOI: 10.1042/BCJ20210724
Mechanisms of TNF-independent RIPK3-mediated cell death
Abstract
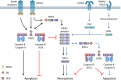
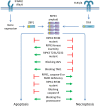
Apoptosis and necroptosis regulate many aspects of organismal biology and are involved in various human diseases. TNF is well known to induce both of these forms of cell death and the underlying mechanisms have been elaborately described. However, cells can also engage apoptosis and necroptosis through TNF-independent mechanisms, involving, for example, activation of the pattern recognition receptors Toll-like receptor (TLR)-3 and -4, or zDNA-binding protein 1 (ZBP1). In this context, cell death signaling depends on the presence of receptor-interacting serine/threonine protein kinase 3 (RIPK3). Whereas RIPK3 is required for TNF-induced necroptosis, it mediates both apoptosis and necroptosis upon TLR3/4 and ZBP1 engagement. Here, we review the intricate mechanisms by which TNF-independent cell death is regulated by RIPK3.
Keywords: RIPK3; apoptosis; cell death; immunology; inflammation; necroptosis.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with this manuscript. D.R.G. consults for Inzen Pharnaceuticals, Ventus Pharmaceuticals, and Boehringer-Ingleheim.
Figures
Similar articles
-
Cell-type dependence of necroptosis pathways triggered by viral infection.FEBS J. 2024 Jun;291(11):2388-2404. doi: 10.1111/febs.17045. Epub 2024 Jan 9. FEBS J. 2024. PMID: 38145501
-
c-Jun N-terminal kinases differentially regulate TNF- and TLRs-mediated necroptosis through their kinase-dependent and -independent activities.Cell Death Dis. 2018 Nov 15;9(12):1140. doi: 10.1038/s41419-018-1189-2. Cell Death Dis. 2018. PMID: 30442927 Free PMC article.
-
FKBP12 mediates necroptosis by initiating RIPK1-RIPK3-MLKL signal transduction in response to TNF receptor 1 ligation.J Cell Sci. 2019 May 20;132(10):jcs227777. doi: 10.1242/jcs.227777. J Cell Sci. 2019. PMID: 31028177
-
The Inflammatory Signal Adaptor RIPK3: Functions Beyond Necroptosis.Int Rev Cell Mol Biol. 2017;328:253-275. doi: 10.1016/bs.ircmb.2016.08.007. Epub 2016 Sep 22. Int Rev Cell Mol Biol. 2017. PMID: 28069136 Free PMC article. Review.
-
Initiation and execution mechanisms of necroptosis: an overview.Cell Death Differ. 2017 Jul;24(7):1184-1195. doi: 10.1038/cdd.2017.65. Epub 2017 May 12. Cell Death Differ. 2017. PMID: 28498367 Free PMC article. Review.
Cited by
-
BAK contributes critically to necrosis and infarct generation during reperfused myocardial infarction.J Mol Cell Cardiol. 2023 Nov;184:1-12. doi: 10.1016/j.yjmcc.2023.09.004. Epub 2023 Sep 12. J Mol Cell Cardiol. 2023. PMID: 37709008 Free PMC article.
-
Bypassing the guardian: regulated cell death pathways in p53-mutant cancers.Cell Mol Biol Lett. 2025 Jun 14;30(1):68. doi: 10.1186/s11658-025-00751-5. Cell Mol Biol Lett. 2025. PMID: 40517236 Free PMC article. Review.
-
Receptor-Interacting Protein Kinase 3-Mediated Modulation of Endothelial Cell Necroptosis and Mitochondrial Dysfunction through AMPK/Drp1 Signaling Pathway: Insights into the Pathophysiological Mechanisms of Lipopolysaccharide-Induced Acute Lung Injury.Int J Med Sci. 2025 Jan 1;22(1):71-86. doi: 10.7150/ijms.104932. eCollection 2025. Int J Med Sci. 2025. PMID: 39744171 Free PMC article.
-
The interaction between RIPK1 and FADD controls perinatal lethality and inflammation.Cell Rep. 2024 Jun 25;43(6):114335. doi: 10.1016/j.celrep.2024.114335. Epub 2024 Jun 8. Cell Rep. 2024. PMID: 38850531 Free PMC article.
-
RHIMoving fibrils of death.Cell Res. 2023 Nov;33(11):811-812. doi: 10.1038/s41422-023-00875-3. Cell Res. 2023. PMID: 37700166 Free PMC article. No abstract available.
References
-
- Laster, S.M., Wood, J.G. and Gooding, L.R. (1988) Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J. Immunol. 141, 2629–2634 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

