Long-term Effectiveness and Safety of Rituximab in Neuromyelitis Optica Spectrum Disorder and MOG Antibody Disease
- PMID: 36240094
- PMCID: PMC9728038
- DOI: 10.1212/WNL.0000000000201260
Long-term Effectiveness and Safety of Rituximab in Neuromyelitis Optica Spectrum Disorder and MOG Antibody Disease
Abstract
Background and objectives: Rituximab is used widely for relapse prevention in neuromyelitis optica spectrum disorder (NMOSD) and myelin oligodendrocyte glycoprotein (MOG)-IgG-associated disease (MOGAD); however, data regarding the effectiveness and safety of long-term rituximab use in these conditions are limited. In this study, we sought to evaluate long-term clinical outcomes in patients with aquaporin-4 IgG-seropositive (AQP4-IgG+) NMOSD and MOGAD treated with rituximab.
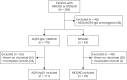
Methods: We performed a retrospective chart review of patients with AQP4-IgG+ NMOSD or MOGAD followed at the Johns Hopkins Neuromyelitis Optica Clinic and included patients who had received at least 1 dose of rituximab.
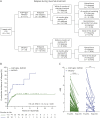
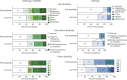
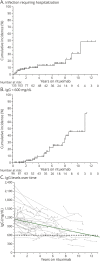
Results: We identified 111 patients with NMOSD and 23 patients with MOGAD who fulfilled the inclusion criteria. The median duration of rituximab treatment for the patients with NMOSD was 3.7 years (range: 0.5-13.2 years) and for the patients with MOGAD was 2.1 years (range: 0.5-7.0 years). The annualized relapse rate (ARR) decreased after rituximab initiation in both NMOSD (median ARR: pretreatment 1.1, posttreatment 0; p < 0.001) and MOGAD (median ARR: pretreatment 1.9, posttreatment 0.3; p = 0.002). Relapses on rituximab occurred in 31 patients with NMOSD (28%) and 14 patients with MOGAD (61%). The majority of NMOSD treatment failures (37/48 relapses; 77%) occurred either within the initial 6 months after starting rituximab (n = 13 relapses) or in the setting of delayed/missed rituximab doses and/or peripheral B-cell reconstitution (n = 24 relapses), whereas in MOGAD, these circumstances were present in a smaller proportion of treatment failures (19/35 relapses; 54%). The risk of relapse on rituximab was greater for patients with MOGAD compared with patients with NMOSD (hazard ratio: 2.8, 95% CI: 1.5-5.2, p = 0.001). Infections requiring hospitalization occurred in 13% and immunoglobulin G (IgG) hypogammaglobulinemia in 17% of patients. The median rituximab treatment duration before IgG hypogammaglobulinemia onset was 5.4 years (interquartile range: 3.8-7.7 years).
Discussion: Rituximab treatment is associated with the reduced annualized relapse rate in AQP4-IgG-seropositive NMOSD, especially in the absence of gaps in treatment and/or B-cell reconstitution. In MOGAD, although a reduction in relapses was observed after initiation of rituximab, this association appeared to be less robust than in AQP4-IgG-seropositive NMOSD. Severe infections and hypogammaglobulinemia occurred in a significant proportion of patients, highlighting the need for close monitoring of infectious complications.
Classification of evidence: This study provides Class IV evidence that rituximab decreases the annualized relapse rate in AQP4-IgG-seropositive NMOSD and MOGAD.
© 2022 American Academy of Neurology.
Figures

References
-
- Marignier R, Hacohen Y, Cobo-Calvo A, et al. . Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. 2021;20(9):762-772. - PubMed
-
- Flanagan EP. Neuromyelitis optica spectrum disorder and other non-multiple sclerosis central nervous system inflammatory diseases. Continuum (Minneap Minn). 2019;25(3):815-844. - PubMed
-
- Carnero Contentti E, Rojas JI, Cristiano E, et al. . Latin American consensus recommendations for management and treatment of neuromyelitis optica spectrum disorders in clinical practice. Mult Scler Relat Disord. 2021;52:103026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
