Endothelial derived miRNA-9 mediated cardiac fibrosis in diabetes and its regulation by ZFAS1
- PMID: 36240130
- PMCID: PMC9565427
- DOI: 10.1371/journal.pone.0276076
Endothelial derived miRNA-9 mediated cardiac fibrosis in diabetes and its regulation by ZFAS1
Abstract
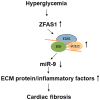
Diabetic cardiomyopathy (DCM) is one of the most prevalent causes of morbidity and mortality in diabetic patients. Hyperglycemia induces increased expression/deposition of extracellular matrix (ECM) proteins including fibronectin (FN) and collagen (Col) and plays an important role in fibrosis in diabetic cardiomyopathy (DCM). The roles of RNAs including microRNA (miRNA) and long non-coding RNAs (lncRNA) have begun to be understood in many conditions. In this study, we investigated the role of a specific miRNA, miR-9, and its interactions with lncRNA ZFAS1 in mediating fibrosis in DCM. Treatment with 25 mM glucose (HG) decreased miR-9 expression and increased expressions of ZFAS1, ECM proteins and inflammatory markers, compared to 5 mM glucose (NG) in the HCMECs by using qRT-PCR. Glucose-induced upregulation of ECM proteins can be prevented by ZFAS1 siRNA or miR-9 mimic transfection. Luciferase assay was confirmed miR-9 binding to FN 3'-UTR. miR-9 expression can be regulated by ZFAS1 through polycomb repressive complex 2 (PRC2) components using RNA immunoprecipitation (RIP) and chromatin immunoprecipitation (ChIP) assays. In the in vivo experiment, hyperglycemia-induced the ECM production can be prevented by the miR-9 overexpression in the fibrosis in DCM. These studies showed a novel glucose-induced molecular mechanism in which ZFAS1 participates in the transcriptional regulation of ECM protein production in diabetes through miR-9.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
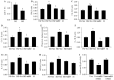
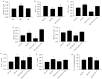
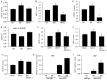
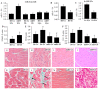
Similar articles
-
Inhibition of the long non-coding RNA ZFAS1 attenuates ferroptosis by sponging miR-150-5p and activates CCND2 against diabetic cardiomyopathy.J Cell Mol Med. 2021 Nov;25(21):9995-10007. doi: 10.1111/jcmm.16890. Epub 2021 Oct 5. J Cell Mol Med. 2021. PMID: 34609043 Free PMC article.
-
miR-146a-Mediated extracellular matrix protein production in chronic diabetes complications.Diabetes. 2011 Nov;60(11):2975-84. doi: 10.2337/db11-0478. Epub 2011 Sep 1. Diabetes. 2011. PMID: 21885871 Free PMC article.
-
LncRNA ZFAS1 regulates the proliferation, oxidative stress, fibrosis, and inflammation of high glucose-induced human mesangial cells via the miR-588/ROCK1 axis.Diabetol Metab Syndr. 2022 Jan 28;14(1):21. doi: 10.1186/s13098-022-00791-3. Diabetol Metab Syndr. 2022. PMID: 35090549 Free PMC article.
-
MicroRNAs and long non-coding RNAs in the pathophysiological processes of diabetic cardiomyopathy: emerging biomarkers and potential therapeutics.Cardiovasc Diabetol. 2021 Feb 27;20(1):55. doi: 10.1186/s12933-021-01245-2. Cardiovasc Diabetol. 2021. PMID: 33639953 Free PMC article. Review.
-
MicroRNA targets and biomarker validation for diabetes-associated cardiac fibrosis.Pharmacol Res. 2021 Dec;174:105941. doi: 10.1016/j.phrs.2021.105941. Epub 2021 Oct 14. Pharmacol Res. 2021. PMID: 34656765 Review.
Cited by
-
CircRNA_012164/MicroRNA-9-5p axis mediates cardiac fibrosis in diabetic cardiomyopathy.PLoS One. 2024 Jul 23;19(7):e0302772. doi: 10.1371/journal.pone.0302772. eCollection 2024. PLoS One. 2024. PMID: 39042659 Free PMC article.
-
Potential Involvement of LncRNAs in Cardiometabolic Diseases.Genes (Basel). 2023 Jan 13;14(1):213. doi: 10.3390/genes14010213. Genes (Basel). 2023. PMID: 36672953 Free PMC article. Review.
-
Noncoding RNAs as Key Regulators for Cardiac Development and Cardiovascular Diseases.J Cardiovasc Dev Dis. 2023 Apr 12;10(4):166. doi: 10.3390/jcdd10040166. J Cardiovasc Dev Dis. 2023. PMID: 37103045 Free PMC article. Review.
-
Epigenetic modifier alpha-ketoglutarate modulates aberrant gene body methylation and hydroxymethylation marks in diabetic heart.Epigenetics Chromatin. 2023 Apr 27;16(1):12. doi: 10.1186/s13072-023-00489-4. Epigenetics Chromatin. 2023. PMID: 37101286 Free PMC article.
-
MicroRNA 9 Is a Regulator of Endothelial to Mesenchymal Transition in Diabetic Retinopathy.Invest Ophthalmol Vis Sci. 2023 Jun 1;64(7):13. doi: 10.1167/iovs.64.7.13. Invest Ophthalmol Vis Sci. 2023. PMID: 37279396 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

