Analysis of disparities in time to allogeneic transplantation in adults with acute myelogenous leukemia
- PMID: 36240477
- PMCID: PMC10393759
- DOI: 10.1182/bloodadvances.2022008572
Analysis of disparities in time to allogeneic transplantation in adults with acute myelogenous leukemia
Abstract
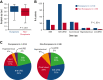
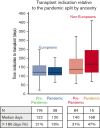
Although alternative donors extend transplant access, whether recipient ancestry affects the time to allogeneic transplant is not established. We analyzed the likelihood of clinically significant delays to allograft by patient ancestry in 313 adult patients with acute myelogenous leukemia (AML) who underwent transplantation. Non-European ancestry patients (n = 99) were more likely than Europeans (n = 214) to receive HLA-mismatched donor allografts (45% vs 24%). Overall, the median time from transplant indication to allograft was 127 days (range, 57-1683). In multivariable analysis, non-Europeans had an increased risk of prolonged indication to transplant time >180 days owing to significant delays in indication to consult >90 days and consult to transplant >120 days. Compared with recipients of HLA-matched unrelated donors (URDs), HLA-mismatched adult donor recipients were at an increased risk of delayed indication to transplant, whereas HLA-identical sibling and cord blood recipients were at a lower risk. Subanalysis showed more indication to transplant delays >180 days in non-European (44%) vs European (19%) 8/8 URD recipients. Finally, the pandemic further exacerbated delays for non-Europeans. In summary, although non-European patients with AML are less likely to receive 8/8 URDs as expected, if they do, their transplants are delayed. HLA-identical siblings and cord blood facilitate the fastest transplants regardless of patient ancestry, whereas other adult donor transplants are delayed. Strategies to mitigate referral barriers, hasten donor evaluation, and use all alternative donor sources are critical to ensure timely transplantation for patients with AML.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: B.G. has received research funding from Actinium Pharmaceuticals and serves on the data and safety and monitoring board for Synthetic Biologics, Inc. A.S. serves as a consultant at the Scientific Advisory Board of ExCellThera. S.A.G. has served as a consultant for Amgen, Actinium, Celgene, Johnson & Johnson, Jazz Pharmaceutical, Takeda, Novartis, Kite, and Spectrum Pharma and has received research funding from Amgen, Actinium, Celgene, Johnson & Johnson, Miltenyi, and Takeda. M.-A.P. has received honoraria from AbbVie, Bellicum, Bristol-Myers Squibb, Incyte, Kite (Gilead), Merck, Novartis, Nektar Therapeutics, and Takeda; serves on data and safety monitoring boards for Servier, Cidara Therapeutics and Medigene; serves on the scientific advisory boards of MolMed and NexImmune; and has received research support for clinical trials from Incyte, Kite (Gilead), and Miltenyi Biotec. C.S.S. has served as a paid consultant on advisory boards for Juno Therapeutics, Sanofi-Genzyme, Spectrum Pharmaceuticals, Novartis, Genmab, Precision Biosciences, Kite (Gilead), Celgene, Gamida Cell, and GlaxoSmithKline and has received research funds for clinical trials from Juno Therapeutics, Celgene, Precision Biosciences, and Sanofi-Genzyme. J.W.Y. owns equity in Merck, Pfizer, and Amgen. I.P. has received research funding from Merck and serves as a member on a Data and Safety Monitoring Board for ExCellThera. J.N.B. has received consultancy payments from Gamida Cell and the New York Blood Center and a research funding from Merck. The remaining authors declare no competing financial interests.
Figures


References
-
- Rashidi A, Slade M, DiPersio J, et al. Post-transplant high-dose cyclophosphamide after HLA-matched vs haploidentical hematopoietic cell transplantation for AML. Bone Marrow Transplant. 2016;51(12):1561–1564. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

