The gut peptide Reg3g links the small intestine microbiome to the regulation of energy balance, glucose levels, and gut function
- PMID: 36240758
- PMCID: PMC9633559
- DOI: 10.1016/j.cmet.2022.09.024
The gut peptide Reg3g links the small intestine microbiome to the regulation of energy balance, glucose levels, and gut function
Abstract
Changing composition of the gut microbiome is an important component of the gut adaptation to various environments, which have been implicated in various metabolic diseases including obesity and type 2 diabetes, but the mechanisms by which the microbiota influence host physiology remain contentious. Here we find that both diets high in the fermentable fiber inulin and vertical sleeve gastrectomy increase intestinal expression and circulating levels of the anti-microbial peptide Reg3g. Moreover, a number of beneficial effects of these manipulations on gut function, energy balance, and glucose regulation are absent in Reg3g knockout mice. Peripheral administration of various preparations of Reg3g improves glucose tolerance, and this effect is dependent on the putative receptor Extl3 in the pancreas. These data suggest Reg3g acts both within the lumen and as a gut hormone to link the intestinal microbiome to various aspects of host physiology that may be leveraged for novel treatment strategies.
Keywords: Reg3g, obesity, type 2 diabetes, gut function, gut microbiome.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests R.J.S. has received research support from Novo Nordisk, Astra Zeneca, Pfizer, and Fractyl. R.J.S has served as a paid consultant and/or scientific advisory board member to Novo Nordisk, Scohia, Fractyle and ShouTi Pharma. R.J.S. has equity or option positions in Rewind and Calibrate Health. S.L.-A. is a paid employee of Novo Nordisk.
Figures
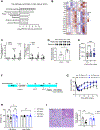





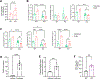
Comment in
-
Out of destruction comes new growth: Pore-forming antimicrobials make pancreas grow.Cell Metab. 2022 Nov 1;34(11):1611-1613. doi: 10.1016/j.cmet.2022.10.006. Cell Metab. 2022. PMID: 36323229
References
-
- Arble DM, Evers SS, Bozadjieva N, Frikke-Schmidt H, Myronovych A, Lewis A, Toure MH, and Seeley RJ (2018). Metabolic comparison of one-anastomosis gastric bypass, single-anastomosis duodenal-switch, Roux-en-Y gastric bypass, and vertical sleeve gastrectomy in rat. Surg Obes Relat Dis 14, 1857–1867. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U24 DK097771/DK/NIDDK NIH HHS/United States
- T32 DK108740/DK/NIDDK NIH HHS/United States
- R01 DK107282/DK/NIDDK NIH HHS/United States
- P01 DK117821/DK/NIDDK NIH HHS/United States
- R01 DK133140/DK/NIDDK NIH HHS/United States
- R01 DK107652/DK/NIDDK NIH HHS/United States
- P30 DK034933/DK/NIDDK NIH HHS/United States
- R01 DK121995/DK/NIDDK NIH HHS/United States
- UL1 TR002240/TR/NCATS NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- U2C DK110768/DK/NIDDK NIH HHS/United States
- P30 DK089503/DK/NIDDK NIH HHS/United States
- IK2 BX005715/BX/BLRD VA/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

