Evaluating the effect of measles and rubella mass vaccination campaigns on seroprevalence in India: a before-and-after cross-sectional household serosurvey in four districts, 2018-2020
- PMID: 36240831
- PMCID: PMC9579355
- DOI: 10.1016/S2214-109X(22)00379-5
Evaluating the effect of measles and rubella mass vaccination campaigns on seroprevalence in India: a before-and-after cross-sectional household serosurvey in four districts, 2018-2020
Abstract
Background: India did phased measles-rubella supplementary immunisation activities (MR-SIAs; ie, mass-immunisation campaigns) targeting children aged 9 months to less than 15 years. We estimated measles-rubella seroprevalence before and after the MR-SIAs to quantify the effect on population immunity and identify remaining immunity gaps.
Methods: Between March 9, 2018 and March 19, 2020 we did community-based, cross-sectional serosurveys in four districts in India before and after MR-SIAs. 30 villages or wards were selected within each district, and one census enumeration block from each was selected as the survey cluster. Households were enumerated and 13 children in the younger age group (9 months to <5 years) and 13 children in the older ager group (5 to <15 years) were randomly selected by use of computer-generated random numbers. Serum samples were tested for IgG antibodies to measles and rubella viruses by enzyme immunoassay.
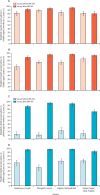
Findings: Specimens were collected from 2570 children before the MR-SIA and from 2619 children afterwards. The weighted MR-SIA coverage ranged from 73·7% to 90·5% in younger children and from 73·6% to 93·6% in older children. Before the MR-SIA, district-level measles seroprevalence was between 80·7% and 88·5% among younger children in all districts, and between 63·4% and 84·5% among older children. After the MR-SIA, measles seroprevalence among younger children increased to more than 90% (range 91·5 to 96·0) in all districts except Kanpur Nagar, in which it remained unchanged 80·4%. Among older children, measles seroprevalence increased to more than 90·0% (range 93·7% to 96·5%) in all districts except Hoshiarpur (88·7%). A significant increase in rubella seroprevalence was observed in all districts in both age groups, with the largest effect in Dibrugarh, where rubella seroprevalence increased from 10·6% to 96·5% among younger children.
Interpretation: Measles-rubella seroprevalence increased substantially after the MR-SIAs but the serosurvey also identified remaining gaps in population immunity.
Funding: The Bill & Melinda Gates Foundation and Indian Council of Medical Research.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests KH has been an employee by Pfizer Vaccines from October 26, 2020. All other authors declare no competing interests.
Figures

References
-
- Ministry of Health and Family Welfare. Government of India National Family Health Survey-1, 1992–93. India main report. August, 1995. http://rchiips.org/NFHS/data/india1/iachap9.pdf
-
- Government of India Ministry of Health and Family Welfare National Family Health Survey—5. 2019–21. India factsheet. November, 2021. http://rchiips.org/nfhs/NFHS-5_FCTS/India.pdf
-
- Gupta SK, Sosler S, Haldar P, Hombergh H, Bose AS. Introduction strategy of a second dose measles containing vaccine in India. Indian Pediatr. 2011;48:379–382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

