Cardiovascular outcomes in adults with hypertension with evening versus morning dosing of usual antihypertensives in the UK (TIME study): a prospective, randomised, open-label, blinded-endpoint clinical trial
- PMID: 36240838
- PMCID: PMC9631239
- DOI: 10.1016/S0140-6736(22)01786-X
Cardiovascular outcomes in adults with hypertension with evening versus morning dosing of usual antihypertensives in the UK (TIME study): a prospective, randomised, open-label, blinded-endpoint clinical trial
Abstract
Background: Studies have suggested that evening dosing with antihypertensive therapy might have better outcomes than morning dosing. The Treatment in Morning versus Evening (TIME) study aimed to investigate whether evening dosing of usual antihypertensive medication improves major cardiovascular outcomes compared with morning dosing in patients with hypertension.
Methods: The TIME study is a prospective, pragmatic, decentralised, parallel-group study in the UK, that recruited adults (aged ≥18 years) with hypertension and taking at least one antihypertensive medication. Eligible participants were randomly assigned (1:1), without restriction, stratification, or minimisation, to take all of their usual antihypertensive medications in either the morning (0600-1000 h) or in the evening (2000-0000 h). Participants were followed up for the composite primary endpoint of vascular death or hospitalisation for non-fatal myocardial infarction or non-fatal stroke. Endpoints were identified by participant report or record linkage to National Health Service datasets and were adjudicated by a committee masked to treatment allocation. The primary endpoint was assessed as the time to first occurrence of an event in the intention-to-treat population (ie, all participants randomly assigned to a treatment group). Safety was assessed in all participants who submitted at least one follow-up questionnaire. The study is registered with EudraCT (2011-001968-21) and ISRCTN (18157641), and is now complete.
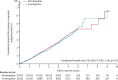
Findings: Between Dec 17, 2011, and June 5, 2018, 24 610 individuals were screened and 21 104 were randomly assigned to evening (n=10 503) or morning (n=10 601) dosing groups. Mean age at study entry was 65·1 years (SD 9·3); 12 136 (57·5%) participants were men; 8968 (42·5%) were women; 19 101 (90·5%) were White; 98 (0·5%) were Black, African, Caribbean, or Black British (ethnicity was not reported by 1637 [7·8%] participants); and 2725 (13·0%) had a previous cardiovascular disease. By the end of study follow-up (March 31, 2021), median follow-up was 5·2 years (IQR 4·9-5·7), and 529 (5·0%) of 10 503 participants assigned to evening treatment and 318 (3·0%) of 10 601 assigned to morning treatment had withdrawn from all follow-up. A primary endpoint event occurred in 362 (3·4%) participants assigned to evening treatment (0·69 events [95% CI 0·62-0·76] per 100 patient-years) and 390 (3·7%) assigned to morning treatment (0·72 events [95% CI 0·65-0·79] per 100 patient-years; unadjusted hazard ratio 0·95 [95% CI 0·83-1·10]; p=0·53). No safety concerns were identified.
Interpretation: Evening dosing of usual antihypertensive medication was not different from morning dosing in terms of major cardiovascular outcomes. Patients can be advised that they can take their regular antihypertensive medications at a convenient time that minimises any undesirable effects.
Funding: British Heart Foundation.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests ISM reports research grants from Menarini, EMA, Sanofi, HDR UK, National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA), and Innovative Medicines Initiative outside of the submitted work; institutional consultancy income from AstraZeneca outside of the submitted work; and personal income from AstraZeneca and Amgen outside of the submitted work. TMM reports grants from Menarini/Ipsen/Teijin, NIHR HTA, and MSD outside of the submitted work; personal income for consultancy from Novartis and AstraZeneca outside of the submitted work; and is a trustee of the Scottish Heart Arterial Risk Prevention (SHARP) Society. NRP reports receiving financial support from several pharmaceutical companies that manufacture blood pressure lowering agents for consulting (Servier and Aktiia), research projects and staffing (Servier and Pfizer), and for arranging and speaking at educational meetings (Servier, Sanofi, Eva Pharma, Pfizer, Emcure India, and Dr Reddy's Laboratories); he holds no stocks or shares in any of these companies. BW is supported by the NIHR University College London Hospitals Biomedical Research Centre; reports research grants from Omron, MRC, and NIHR; and reports honoraria from Daiichi Sankyo, Omron, Servier, Pfizer, Novartis, and Menarini outside of the submitted work. MJB is supported by the NIHR Barts Health Biomedical Research Centre and reports research grants from the British Heart Foundation, MRC, and NIHR. PMR declares consultancy income from Abbot and Bayer, and honoraria from Sanofi; he is also a member of the AXIOMATIC trial Data Monitoring Committee. CCL reports membership of a data safety monitoring board or steering committee for Novo Nordisk. AR reports an unpaid membership of an NIHR steering committee. AM reports a British Heart Foundation grant to his university to support salary costs. All other authors declare no competing interests.
Figures

Comment in
-
Best time for administration of antihypertensive medications: morning or evening?Lancet. 2022 Oct 22;400(10361):1383-1385. doi: 10.1016/S0140-6736(22)01900-6. Epub 2022 Oct 11. Lancet. 2022. PMID: 36240839 No abstract available.
-
Antihypertensive drugs can be taken in the morning or evening.Nat Rev Cardiol. 2023 Jan;20(1):3. doi: 10.1038/s41569-022-00801-x. Nat Rev Cardiol. 2023. PMID: 36319686 No abstract available.
-
In hypertension, evening vs. morning dosing of antihypertensive drugs did not differ for major CV outcomes at 5.2 y.Ann Intern Med. 2023 Feb;176(2):JC16. doi: 10.7326/J22-0116. Epub 2023 Feb 7. Ann Intern Med. 2023. PMID: 36745893
-
Putting night-time dosing of antihypertensives to bed for now.Drug Ther Bull. 2023 Apr;61(4):50. doi: 10.1136/dtb.2022.000069. Epub 2023 Mar 9. Drug Ther Bull. 2023. PMID: 36894302 No abstract available.
References
-
- Fagard RH, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Night-day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J Hum Hypertens. 2009;23:645–653. - PubMed
-
- Salles GF, Reboldi G, Fagard RH, et al. Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: the Ambulatory Blood Pressure Collaboration in patients with Hypertension (ABC-H) meta-analysis. Hypertension. 2016;67:693–700. - PubMed
-
- Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension. 2010;56:765–773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

