Immunopeptidomics-based design of mRNA vaccine formulations against Listeria monocytogenes
- PMID: 36241641
- PMCID: PMC9562072
- DOI: 10.1038/s41467-022-33721-y
Immunopeptidomics-based design of mRNA vaccine formulations against Listeria monocytogenes
Abstract
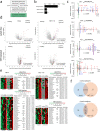
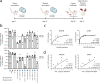
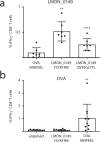
Listeria monocytogenes is a foodborne intracellular bacterial pathogen leading to human listeriosis. Despite a high mortality rate and increasing antibiotic resistance no clinically approved vaccine against Listeria is available. Attenuated Listeria strains offer protection and are tested as antitumor vaccine vectors, but would benefit from a better knowledge on immunodominant vector antigens. To identify novel antigens, we screen for Listeria peptides presented on the surface of infected human cell lines by mass spectrometry-based immunopeptidomics. In between more than 15,000 human self-peptides, we detect 68 Listeria immunopeptides from 42 different bacterial proteins, including several known antigens. Peptides presented on different cell lines are often derived from the same bacterial surface proteins, classifying these antigens as potential vaccine candidates. Encoding these highly presented antigens in lipid nanoparticle mRNA vaccine formulations results in specific CD8+ T-cell responses and induces protection in vaccination challenge experiments in mice. Our results can serve as a starting point for the development of a clinical mRNA vaccine against Listeria and aid to improve attenuated Listeria vaccines and vectors, demonstrating the power of immunopeptidomics for next-generation bacterial vaccine development.
© 2022. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: R.L.M. and F.I. are inventors and R.V., C.A., I.A., S.D.S. and I.L. are contributors to patent application no. EP22170845.6, Vaccine Compositions against
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

